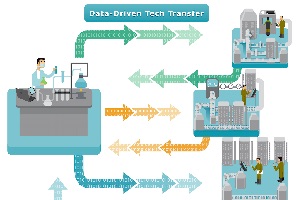
Effective Tech Transfer is crucial to delivering innovative products to market. Can companies improve Tech Transfer through the use of digital data, processes, and technology? We surveyed over 200 companies to find out. Please enjoy the summary below. For the full report, please visit our sponsor BIOVIA Dassault Systems (registration required). Improve Tech Transfer, Improve Profitability…
Recipe
The CPG Digital Thread (infographic)
This infographic shares the importance of developing a cohesive digital thread when developing formulated products in the consumer packaged goods industry. The digital thread should be driven by customer requirements and incorporate the recipe / formulation, specifications, packaging design, compliance information, claims, cautions, ingredients, labeling, artwork, and more to provide a full view of the product…
Enginuity Transformed to Enovia and the 3DExperience Platform
Formulation systems just took a major step forward in the enterprise. I visited Dassault Systemes in Waltham, Massachusetts to get a look at the latest version of the Enginuity formulation system. DS has made a lot of progress integrating Enginuity into their PLM suite and showed me Enginuity is now a full part of Enovia. Why…
Dassault Systemes Acquires the Recipe for Developing Formula-Based Products with Enginuity
I had the chance to talk with … Dassault Systemes recently about acquiring process PLM vendor Enginuity PLM, see the announcement here. As soon as I saw my friend Dr. John Sottery‘s name on the webcast along with Enovia CEO Mich Tellier, I had a pretty good guess about what they were going to tell…
Product Compliance – The Hidden Tax on Innovation
Issue in Focus: Product Compliance – The Hidden Tax on Innovation: Enhancing Innovation in Formula-Based Companies through Real-time, Automated Compliance Monitoring discusses the need for early product compliance checking for formula- and recipe-based products. Explains how manual processes for compliance checking and documentation limit product innovation, and how PLM with compliance checking built in can…
Product Compliance – Hidden Tax on Product Innovation
A quick peek into some research on product compliance for formula-based companies. Formula- and recipe-based product developers face their own set of compliance challenges. This report focused on their needs, and how PLM systems can help reduce the manual workload burden placed on them by product compliance.