Can pharmaceutical and life sciences manufacturers get simplified management by exception in production? Can they get a proven and full-function GMP-ready MES that’s also configurable yet valuable and out of the box? Decades into its existence, the POMS Corporation team says their POMSnet Aquila SaaS solution can do all that. Their satisfied and committed customer base helps us believe they are right.
Both/And, not Either/Or
Comprehensive, configurable, and out-of-the-box are the three pillars of POMSnetAquila MES. This is an unusual combination, as a comprehensive MES is often not ready to use or deliver value out-of-the-box but needs extensive modeling or customization. Many out-of-the-box MES offerings are not very configurable. Configurability is crucial for MES, as every plant and often every area of a plant is a little different.
POMS and its products have won numerous awards over many years. One that stood out to us recognized 97% customer satisfaction. The company has customers large and small. POMS must have vital ongoing customer relationships and an effective approach to incorporating their feedback into the standard product to keep them all happy.
Deep Life Sciences Expertise
For over three decades, POMS has focused its MES on only pharmaceutical, biotech, cell and gene therapy, and as those companies have expanded, more recently, medical device manufacturing. As a result, the solution has comprehensive functionality and is focused entirely on the needs of these regulated industries. POMSnet Aquila has end-to-end GMP manufacturing capabilities that include quality, scheduling, equipment, and production. (The full ISA95 model.)
Automatic recipe generation with a single click is a stunning example of deep functionality. If you manage specifications outside of MES, an integration layer can pull that data into a parameterized template. Attach the master data to the template, and it auto-generates a recipe. The manufacturer can choose to add a review, but this S88-compliant recipe is basically ready to go. One POMS customer with personalized products has one single recipe that’s transformed through the levels from general to site to master to control recipes per batch or patient.

Configured, not Customized
Configurability is increasingly common, but the POMSnet approach is a bit different. Deeply composable, the company has building blocks for recipes and workflows. The company offers 102 pre-built, pre-validated phases or process steps available. It also integrates with these industries’ most common applications and equipment types. Configuring within pre-determined limits helps regulated companies with validation and rapid time to value.
POMSnet Aquila is designed for customers to self-deploy and self-install, for independent customers to expand as needed. The software’s visual nature and pre-built library of over 100 validated manufacturing steps mean you can understand what the software is doing without having to build or model common life sciences processes yourself, such as calculations, weigh and dispense, assays, etc.
Still Going Strong
POMS was a brand in the early days of MES, and now the product is available as cloud-based or on-premise. The UI is any browser with HTML5. The products are on a 2X/year agile release schedule, spring and fall. Each release typically has 80 new features, functions, and UI improvements, most of which come from customer innovation to update the product.
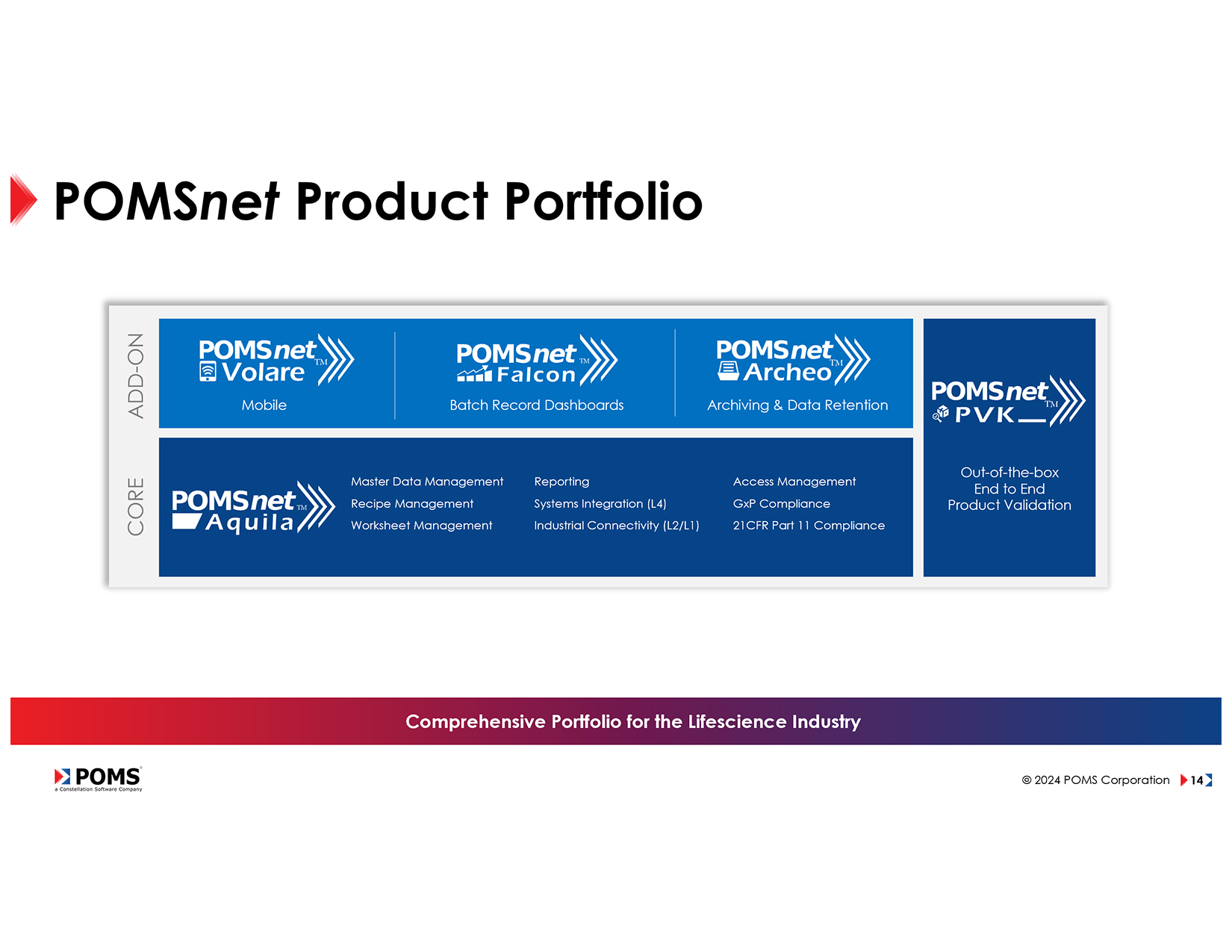
Add-on Options
In addition to the core POMSnet Aquila MES, the product suite includes POMSnet Volare for mobile, POMSnet Falcon for batch record dashboards, and POMSnet Archeo for archiving and data retention. POMS also offers a product validation kit for OQ validating all POMS features. Customers are responsible for PQ, and if it’s on prem IQ; POMS provides IQ for cloud implementations. POMS also offers audit defense, where their quality manager speaks on-site to regulatory offices on behalf of customers.
POMS also partners with Nymi biometrics to provide on-body automatic authentication. This proven device can streamline operators’ interactions with software and create higher levels of confidence in processes, employees’ credentials, and productivity with always-on biometric-based tracking.
Delivering Value Quickly
POMS takes a fast rollout approach with incremental expansion. Since the solution is comprehensive, each customer can start small and expand as they are ready. This enables the validation of smaller processes and data flows to support them. The result is a lower-risk, faster path toward process and system validation.
The POMSnet Falcon Electronic Batch Record Dashboard is not just another PDF or digitized paper. It delivers that and all of the curated GMP data to support review by exception. With a real-time interface, people can see all exceptions, review, approve (or not), and comment across one or multiple production sites. This data-rich environment means backward genealogy is in the system, 1-click away – in a list or chart form. Of course, you can export that to Excel, but it’s data, not a document.
Moving Ahead
With a strong product, happy customers, and an excellent team, we expect to see POMS continue its market growth. As part of Constellation Software Inc., it has had a solid financial footing and framework for vertical market growth.
Thank you, Patrick Nazzaro, President and GM; Roland Esquivel, VP Global Sales & Marketing; and Prashant Jagarlapudi, VP Product and Engineering, for taking the time to get us back up to speed on POMS. We look forward to watching more life sciences companies benefit from this flexible and rapid approach to MES.