We were recently alerted to a company we weren’t covering because a long-time business friend took a key position there. After a quick review of what they do, Julie Fraser and Jim Brown decided to team up on the briefing because we could already see a combination of QMS and PLM capabilities. We learned that there was even more there than we initially thought, with some capabilities that you might expect from ERP and MES systems.
Yet, Enlil is not trying to be all those things to all people. Instead, they aim to be an integrated solution that supports the core needs of a specific audience, the early and mid-stage medical device ecosystem of product developers and contract manufacturers. Their sweet spot, by design, is supporting the varied and nuanced needs of medical device companies as they scale and seek regulatory clearance or approval. They’ve also discovered that contract manufacturers in the life sciences ecosystem find value in what they do. Enlil’s mission is to “empower MedTech product innovators to bring life-changing products to market faster, and with unwavering regulatory confidence.”
The Value of Specialized Solutions
Let’s step back to talk about product specialization. We talk about specialized solutions because nobody likes to be called a niche offering. They feel “niche” limits their potential. Regardless of what you call it, solutions built for a specific customer profile can be immensely valuable because they just fit the customers’ needs. Enlil, Inc. positions itself as a vertical SaaS solution for regulated products. In this case, it’s intrinsic to where Enlil came from and their mission. We feel it’s led to a unique offering with an excellent fit for smaller teams, whether they are startups or focused teams in larger enterprises, focused on developing pre-clinical, class 2 and 3 medical devices.
How Enlil Came to Be
The history is important. Enlil was not created as a startup software company looking for a problem to solve. Instead, Enlil is a purpose-built solution that grew out of an innovation hub that is part of the Shifamed Group. In this scenario, their first customers were their sister companies in the innovation accelerator. This not only helped create focus, but also helped ensure that user needs were met across a variety of similar startup medical device businesses. The result is that they are unique because of where they come from, who they serve, and what they do. Now, they have assembled an impressive team of industry veterans from the ERP, PLM, and MES industries to help grow the business.
What is Enlil?
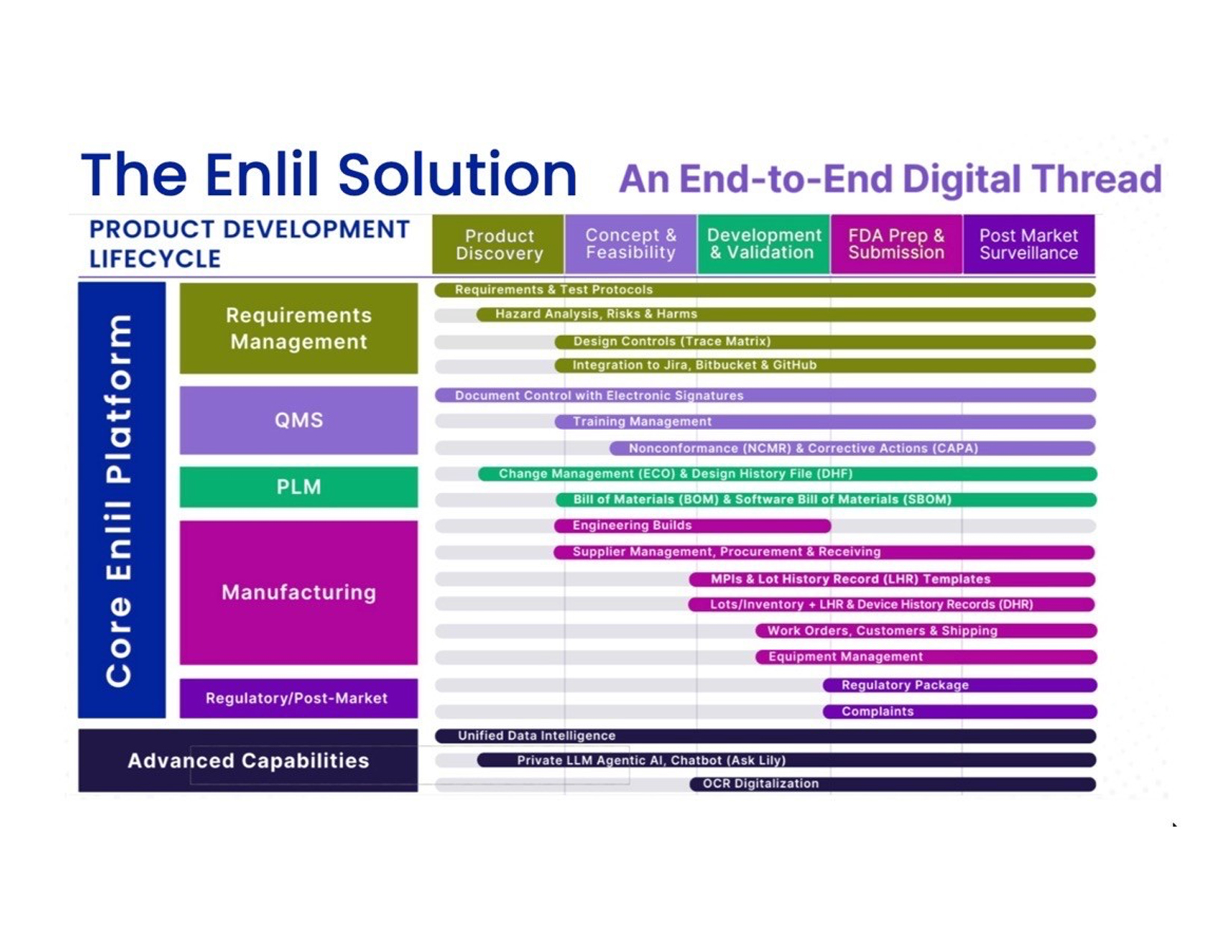
The best way to answer what Enlil is, is by who they support. It’s not a cookie-cutter product that was designed to meet the top ten features of PLM, QMS, MES, or another software category. What we saw was an end-to-end, collaborative product development and innovation system that supports digital thread traceability for medical devices. That’s hard to fit on a marketing slide, but the customer profile focus is the value. What they’ve done is identify the essential needs of their sister companies to use as guiding requirements for the solution.
That leads to an interesting solution that handles QMS functions like traceability and documentation. They can document test results, but they’re not trying to be a LIMS. They manage the digital thread across both hardware and software components, but they aren’t a full PLM system. They manage materials and clinical supply, but they don’t want to become an ERP system. That makes Enlil hard to classify. Are they a PLM with some MES capabilities, a QMS with some PLM functionality? But trying to classify them misses the point. Enlil is a specialized solution tailored to meet the product innovation and product development needs of early-stage medical device developers and their ecosystem.
Who Enlil Targets
As mentioned, Enlil is a specialized offering. The customers that they see as the best bit for their capabilities are these profiles in the medical device ecosystem:
- Small and mid-sized MedTech companies
- Innovation hubs
- MedTech contract manufacturers
They have specialized capabilities that make these companies a good fit, including an interesting two-tier structure that allows innovation hubs, parent companies, or OEMs to share information and collaborate with related entities like incubated startups, product teams, or contract manufacturers. They’re also planning to release an AI chatbot, “Lilly,” to summarize information and help reduce the significant amount of manual work companies spend on regulatory documentation.
There is room for growth beyond these targets, for example, smaller development teams in larger enterprises, but the market they target today is a rich and exciting one. And, one that should appreciate Enlil’s focus.
Our Take
This writeup doesn’t share a lot of details about what Enlil does. Unapologetically, that is because their feature list isn’t what’s important, it’s their specialization on a target profile. Medical device companies looking for a single solution to meet their basic product development and traceability needs should take a look at Enlil. Startups in the medical device industry, who are looking to develop their products and get regulatory approval from the FDA should definitely see how Enlil could help them. It’s rare to find such a tailored solution for a specific set of customers to solve a specific collection of problems. Enlil may not stop there, but they are positioned well to get a strong foothold in early medical device development.
Thank You
Thank you Nader Fathi, Andrew Robbie , Christine Pearsall for introducing us to Enlil and your unique offering. We are excited to learn more and hear from the companies using your solution.