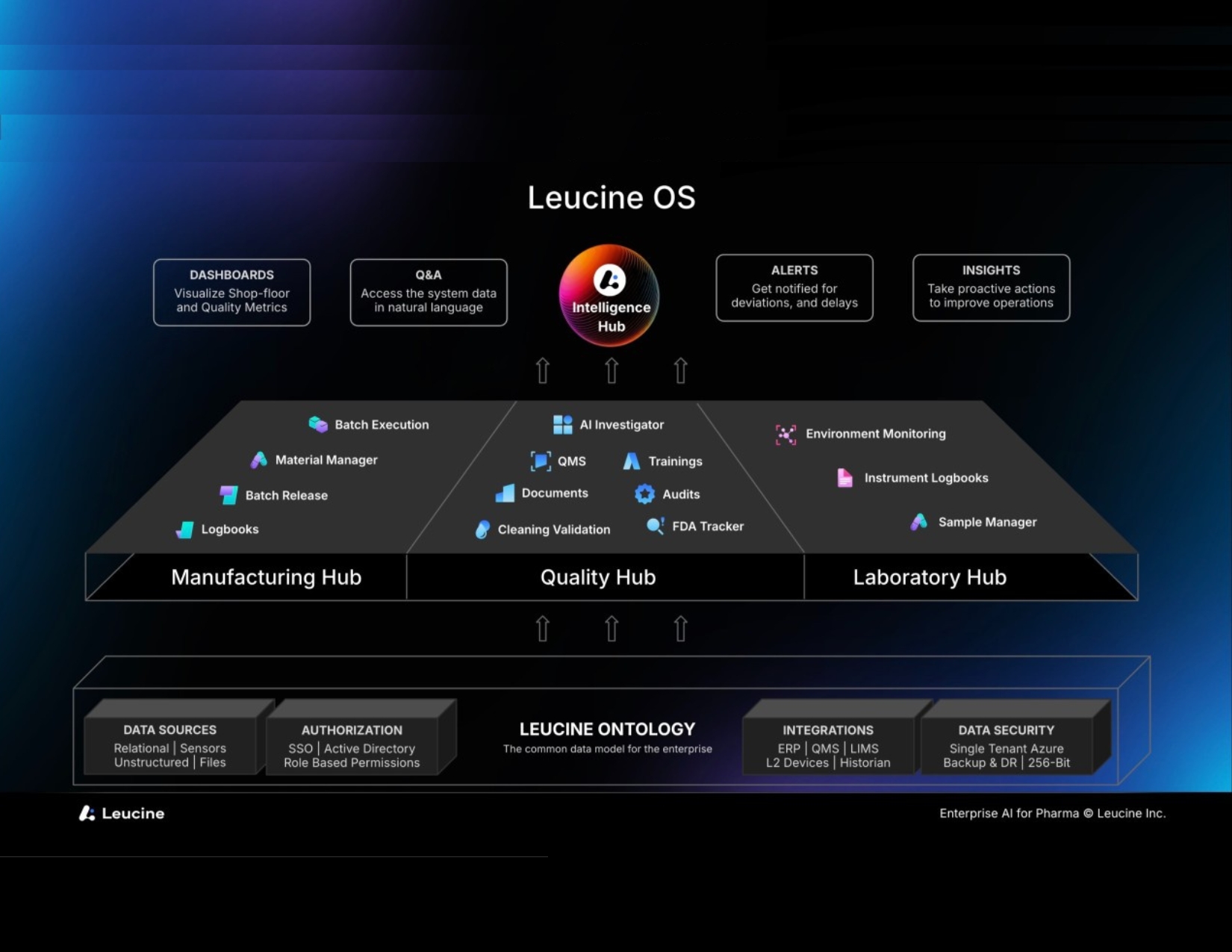
What do pharmaceutical manufacturers most need? To be compliant with government regulations without wasting time and effort. Leucine set out to deliver that with its SaaS software suite, which includes hubs for Manufacturing, Quality, and, most recently, Laboratory, with Intelligence (including AI) serving it all. The company launched in 2019 and joined forces with Ecolab to sell in the US and Europe in 2022. In late 2023, Ecolab, an established cleaning and contamination control provider in the pharmaceutical industry with a digital arm, invested in Leucine.
Software Scope
Leucine is determined to stay focused on manufacturing and quality software for pharmaceutical companies. They aim to help customers achieve two goals:
- Being FDA compliant
- Complying efficiently
That may sound straightforward. Yet, as they point out, most processes are inherently multidisciplinary, so the Leucine suite includes functions for manufacturing, quality, and quality lab activities. On top of the specific functional hubs sits the Intelligence Hub for dashboards, natural language Q&A based on GenAI, alerts, and insights. The Cortex Command Center includes GenAI for goal-based analytics and is typically used by process engineers, production supervisors and operational excellence professionals. Beneath is a common platform to ensure data is shared and in full context across all disciplines.
Manufacturing Hub
In the Manufacturing Hub, where our briefing focused, Leucine’s MES includes:
- Pre-assembled batch recipes with built-in compliance that go all the way to drug-modality specific unit operations such as dispensing, granulation, fermentation and filtration, with weigh and dispense connected via OCR
- Compliant batch execution with over 300 process interlocks and over 1,000 data validations, guided tasks and flows, automated deviation alerts, and escalations to support compliance by design
- WIP material management with two-way reconciliation between ERP and MES, with QR and bar code scanning for lower inventory costs
- Integrated logbooks with automatic time stamps, and not just basics for equipment, but all logs needed for a fully compliant eBR in a cleanroom environment, including cleaning, environmental, and calibration interlocks
Any of these elements can be the starting point for a customer, and as they add functional elements, they seamlessly integrate with in-context data from the ontology. They assert that both data and context are dynamic and took on the challenge of that path to evolution and growth, as well as openness and connectivity.
The MES is designed for operators to have minimal friction. It includes hands-free voice-guided workflows, IoT-connected Andon light integration for process-ready signals, and proactive voice and visual alerts. This includes task timers and audible and visible escalation cues.

Keys to Compliant Software Agility
SaaS, with 99.9% uptime plus ongoing upgrades and security capabilities that the cloud offers, is just the start for agility and data flow. This platform has SOC 2 Type 2 Certified data security for privacy, availability, and security. Both Leucine and Ecolab have specialized expertise in FDA-ready audits, software validation and 21 CFR Part 11.
The platform streamlines connecting to varied data sources. It includes enterprise connectors with OPC-UA and SQL, APIs to QMS and LIMS, RFC and FTP to level 2 systems such as data historians, and OPC-UA or MQTT for analytical balances, plus the native logbooks in the system.
From the company’s inception, they developed a Leucine Ontology or single data model for the enterprise. This ontology has a semantic understanding of the objects involved in pharma manufacturing and quality. Increasingly, we find that an ontology is crucial to effective deployment, integration, and long-term value from manufacturing software.
One thing that stood out to us is the company’s inclusion of an FDA Tracker. This tool keeps up to date with the definition of compliance as it changes. Of course, the analytics intelligence hub and Cortex also support continuous improvement and agility.
Strong Partners
Leucine is a US registered company and has already signed up a strong customer base worldwide since its inception six years ago. It claims over 350 GMP sites and nearly 50 pharmaceutical enterprise customers.
Ecolab brings 30 years of digital innovation, 1,400 employees in the digital group, 100,000 connected sites across 40 industries, 1,000,000 connected devices, and over 120 billion data points go through the Ecolab cloud platform every year.
We think Leucine was brilliant to bring in a strong partner as its channel to market. Ecolab has been making its mark in pharmaceuticals for years, and the decontamination and cleaning processes are a clear part of compliant manufacturing and quality.
Thank you to Leucine co-founder and CEO Vivek Gera, plus Ecolab’s Michael Cates, William Goodman, and Sarah Otterstetter for briefing Rick Franzosa and Julie Fraser on this modern approach to pharmaceutical industry production, quality, and compliance.