How can manufacturers use the digital twin and industrial IoT to dramatically improve manufacturing and product performance? This eBook shares the value of the digital twin and IIoT for products and in the plant. Then, it shares some practical examples and advice to get started down the path to improved performance, availability, and product quality….
- Products (equipment, devices, etc.)
- Production (machines, lines, plants)
- Infrastructure (cities, etc.)
- Something wrong with the physical asset that can be corrected
- Something wrong with the virtual model or simulation that leads to new understanding and provides knowledge for future modeling and simulation efforts
Applying Virtual Twins in the Manufacturing Industry
Target the Right Virtual Twins for Manufacturing There are many ways to gain value by applying the digital twin approach in the manufacturing industries. In the plant, the typical ways to create value are from creating twins for products and for production assets. Let’s discuss how to create and drive value from each of these. Develop Virtual Twins of Products
The first way many people think of the digital twin is as a digital model of a product. This typically starts with a 3D CAD model, but also captures much of the information captured in model-based design approaches. For today’s smart products, the model typically incorporates electronic, software, and systems designs.
The virtual product twin can be used to simulate and communicate planned product behavior and predict product characteristics like throughput, torque, energy consumption, or other performance characteristics. To do this, the virtual twin must accurately represent a specific configuration or unit of the product and be kept up to date with changes. The product model should also include production requirements to support a design anywhere / produce anywhere strategy.
Create Virtual Twins of the Plant
One manufacturer’s product may be the equipment in another’s plant. The virtual model concept extends to equipment, work stations, lines, and entire plants. These twins should completely model production at the appropriate level of granularity for their intended purpose.
The twin can also include the virtual twin of products being produced and the operators running the plant.
These models can be used to simulate and optimize production plans, plant layouts, materials flows, and equipment. Manufacturers can use the models to virtually operate the plant all the way down to control code in order to detect collisions, bottlenecks, and analyze throughput. This allows them to optimize production behavior virtually to get designs right before making physical investments.
Finally, virtual twins can be used to develop code for CNC equipment that can be run and validated on virtual PLCs using an approach known as “software in the loop.” This code can then be used for virtual commissioning to speed ramp up and changeovers.
Develop Virtual Twins of Products
The first way many people think of the digital twin is as a digital model of a product. This typically starts with a 3D CAD model, but also captures much of the information captured in model-based design approaches. For today’s smart products, the model typically incorporates electronic, software, and systems designs.
The virtual product twin can be used to simulate and communicate planned product behavior and predict product characteristics like throughput, torque, energy consumption, or other performance characteristics. To do this, the virtual twin must accurately represent a specific configuration or unit of the product and be kept up to date with changes. The product model should also include production requirements to support a design anywhere / produce anywhere strategy.
Create Virtual Twins of the Plant
One manufacturer’s product may be the equipment in another’s plant. The virtual model concept extends to equipment, work stations, lines, and entire plants. These twins should completely model production at the appropriate level of granularity for their intended purpose.
The twin can also include the virtual twin of products being produced and the operators running the plant.
These models can be used to simulate and optimize production plans, plant layouts, materials flows, and equipment. Manufacturers can use the models to virtually operate the plant all the way down to control code in order to detect collisions, bottlenecks, and analyze throughput. This allows them to optimize production behavior virtually to get designs right before making physical investments.
Finally, virtual twins can be used to develop code for CNC equipment that can be run and validated on virtual PLCs using an approach known as “software in the loop.” This code can then be used for virtual commissioning to speed ramp up and changeovers.
Creating the Complete Digital Twin in Manufacturing
 Gain Greater Value from Connected Digital Twins
The value of digital twins specific to manufacturing is greatly enhanced when the virtual and physical twins are connected via the IIoT. Manufacturer operations can produce a lot of data. For example, companies may already have MES and other control data, but they traditionally have trouble unlocking the value from it. The value of this data is made tangible when it’s put into the context of the digital model. Connecting the virtual and physical twins allows companies to compare actual to expected results and behavior of plants, product lines, work cells, machines, and even the products produced. The digital twin helps them gain intelligence from the data instead of simply storing it away.
Connect and Analyze Plant and Product Virtual Twins
Manufacturers can learn a lot from discrepancies between expected performance predicted using the virtual twin and measured performance of the physical twin. The twin defines what “good” looks like in production and what “right” looks like with the products. Variances from simulated values may identify issues in the plant that can be diagnosed and remedied to bring performance back into plan.
A more complete digital approach leverages analytics and the cloud. Analytics creates deeper insights to gain product and process intelligence. Let’s take a look at how the intelligence derived from the digital twin drives tangible business value for manufacturers.
Gain Greater Value from Connected Digital Twins
The value of digital twins specific to manufacturing is greatly enhanced when the virtual and physical twins are connected via the IIoT. Manufacturer operations can produce a lot of data. For example, companies may already have MES and other control data, but they traditionally have trouble unlocking the value from it. The value of this data is made tangible when it’s put into the context of the digital model. Connecting the virtual and physical twins allows companies to compare actual to expected results and behavior of plants, product lines, work cells, machines, and even the products produced. The digital twin helps them gain intelligence from the data instead of simply storing it away.
Connect and Analyze Plant and Product Virtual Twins
Manufacturers can learn a lot from discrepancies between expected performance predicted using the virtual twin and measured performance of the physical twin. The twin defines what “good” looks like in production and what “right” looks like with the products. Variances from simulated values may identify issues in the plant that can be diagnosed and remedied to bring performance back into plan.
A more complete digital approach leverages analytics and the cloud. Analytics creates deeper insights to gain product and process intelligence. Let’s take a look at how the intelligence derived from the digital twin drives tangible business value for manufacturers.
Improve Equipment Performance and Availability
Monitor and Optimize Equipment Performance The first way we’ll examine the business value of the digital twin in the plant is by improving equipment performance. Today’s manufacturers need to get the most out of available production assets. Manufacturers can use the digital twin to monitor equipment and production via the IIoT. They can aggregate, filter, and analyze sensor and control data in the context of the virtual twin to find issues. The resultant information can be shared in dashboards and put into action with alerts so issues like reduced production rates can be addressed before they arise or escalate. Predict and Improve Equipment Availability One of the most promising ways manufacturers improve productivity is through predictive analytics. Instead of maintaining production equipment on estimated service intervals, manufacturers can monitor equipment to identify when it begins to show signs of wear or failure, such as higher temperatures or vibration. But analytics can also find hidden patterns, anomalies, or trends in operational data that may indicate a pending equipment failure. Transitioning to predictive service allows manufacturers to reduce downtime and avoid the cost of unnecessarily taking equipment offline for maintenance when it’s not necessary. Of course, not every issue can be predicted, and in those cases IoT and the digital twin can provide the intelligence needed to improve issue identification, reduce repair time, and analyze root causes. The resulting enhanced productivity from better equipment performance and availability directly drives improved profitability.
Improve Product Quality
 Monitor and Maintain Product Quality
The next way that digital twins offer tangible business value to plants is by improving quality. Manufacturers can use the IIoT to monitor production and compare it to the digital twin to find goods that are trending out of spec. For example, they could compare product tolerances with IIoT feeds from metrology devices to find anomalies. Then, they can use trend charts, histograms, or dashboards with alerts to identify potential quality slippage.
Control and Assure Process Quality
In addition to measuring production output, manufacturers can monitor the production process to build quality in. They can collect KPIs like cycle times or track control parameters to identify when processes aren’t fully in control so they can intervene.
Manufacturers can also use analytics to understand the relationship between variability in production specifications and output to help identify factors that lead to quality slippage. They can use artificial intelligence (AI) to gain insights from a combination of enterprise system data, environmental conditions, operator information, equipment setup, and machine settings. This can help them close the loop between engineering and actual resulting product performance characteristics, for example finding relationships between equipment settings or environmental factors and defects. They can also leverage AI to identify early indicators that production isn’t behaving as expected in order to identify and correct errors in real time.
Monitor and Maintain Product Quality
The next way that digital twins offer tangible business value to plants is by improving quality. Manufacturers can use the IIoT to monitor production and compare it to the digital twin to find goods that are trending out of spec. For example, they could compare product tolerances with IIoT feeds from metrology devices to find anomalies. Then, they can use trend charts, histograms, or dashboards with alerts to identify potential quality slippage.
Control and Assure Process Quality
In addition to measuring production output, manufacturers can monitor the production process to build quality in. They can collect KPIs like cycle times or track control parameters to identify when processes aren’t fully in control so they can intervene.
Manufacturers can also use analytics to understand the relationship between variability in production specifications and output to help identify factors that lead to quality slippage. They can use artificial intelligence (AI) to gain insights from a combination of enterprise system data, environmental conditions, operator information, equipment setup, and machine settings. This can help them close the loop between engineering and actual resulting product performance characteristics, for example finding relationships between equipment settings or environmental factors and defects. They can also leverage AI to identify early indicators that production isn’t behaving as expected in order to identify and correct errors in real time.
Next Steps
The Digital Industrial Revolution Requires Action Today’s manufacturers need to deliver high operational efficiency in order to compete. It’s time for them to digitalize to gain important benefits – agility, productivity, quality, cost, customer satisfaction, and responsiveness – and remain competitive into the future. Manufacturers can apply the digital twin concept to products, plants, or both to drive new levels of quality and productivity. The digital twin and the IIoT are key digitalization enablers with tangible, proven value. Get Started
To get started, manufacturers can choose between a number of valuable initiatives to leverage the digital twin, IIoT, and analytics. For example, they can use the digital twin, IIoT, and analytics to:
Get Started
To get started, manufacturers can choose between a number of valuable initiatives to leverage the digital twin, IIoT, and analytics. For example, they can use the digital twin, IIoT, and analytics to:
- Gain equipment intelligence and improve uptime
- Shift from preventative to predictive maintenance
- Monitor production to rapidly identify and correct quality or productivity issues
- Leverage actual results from the physical twin to improve simulations by feeding the digital twin with observed production scenarios and parameters
- Explore new business models by applying digital twin capabilities to their own products
 To learn more, see the related Buyer's Guide for Retail Predictive Analytics.
[post_title] => Retail Predictive Analytics Solution (Buyer’s Checklist)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => retail-analytics-checklist
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:27:58
[post_modified_gmt] => 2022-11-15 03:27:58
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7884
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[2] => WP_Post Object
(
[ID] => 7877
[post_author] => 2572
[post_date] => 2019-05-21 11:00:18
[post_date_gmt] => 2019-05-21 15:00:18
[post_content] =>
To learn more, see the related Buyer's Guide for Retail Predictive Analytics.
[post_title] => Retail Predictive Analytics Solution (Buyer’s Checklist)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => retail-analytics-checklist
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:27:58
[post_modified_gmt] => 2022-11-15 03:27:58
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7884
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[2] => WP_Post Object
(
[ID] => 7877
[post_author] => 2572
[post_date] => 2019-05-21 11:00:18
[post_date_gmt] => 2019-05-21 15:00:18
[post_content] =>  How can you empower design engineers to make more informed decisions that can help set your products apart from the competition?
Tech-Clarity’s Empowering Design Engineer infographic helps answer this question based on a survey of 195 companies.
The infographic reveals top qualities that will make products more competitive over the next five years. These product qualities depend on good engineering decisions. Yet, 76% rate these decisions as ‘somewhat hard’ to ‘extremely difficult.’
The infographic uncovers a potential approach to make these decisions easier. The infographic also explores how new approaches to simulation may help make CAE a powerful tool for design engineers.
View the full infographic from our sponsor PTC (no registration required).
To learn more, see the related Revolutionizing Simulation for Design Engineers research report.
[post_title] => Empowering Design Engineers
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => design-engineers-infographic
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-20 00:04:55
[post_modified_gmt] => 2024-01-20 05:04:55
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7877
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[3] => WP_Post Object
(
[ID] => 7823
[post_author] => 2
[post_date] => 2019-05-08 10:43:03
[post_date_gmt] => 2019-05-08 14:43:03
[post_content] =>
How can you empower design engineers to make more informed decisions that can help set your products apart from the competition?
Tech-Clarity’s Empowering Design Engineer infographic helps answer this question based on a survey of 195 companies.
The infographic reveals top qualities that will make products more competitive over the next five years. These product qualities depend on good engineering decisions. Yet, 76% rate these decisions as ‘somewhat hard’ to ‘extremely difficult.’
The infographic uncovers a potential approach to make these decisions easier. The infographic also explores how new approaches to simulation may help make CAE a powerful tool for design engineers.
View the full infographic from our sponsor PTC (no registration required).
To learn more, see the related Revolutionizing Simulation for Design Engineers research report.
[post_title] => Empowering Design Engineers
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => design-engineers-infographic
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-20 00:04:55
[post_modified_gmt] => 2024-01-20 05:04:55
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7877
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[3] => WP_Post Object
(
[ID] => 7823
[post_author] => 2
[post_date] => 2019-05-08 10:43:03
[post_date_gmt] => 2019-05-08 14:43:03
[post_content] => BOM Processes Rely on Inadequate Solutions
 Ineffective BOM Processes Cause Disruption
BOM management is critical to connecting design, purchasing, and production across a manufacturing business. Ineffective BOM processes, though, lead to low productivity and costly errors.
With that in mind, why do so many manufacturers rely on substandard BOM management approaches like spreadsheets and email? Is there a better way for companies to support BOM-related processes?
Ineffective BOM Processes Cause Disruption
BOM management is critical to connecting design, purchasing, and production across a manufacturing business. Ineffective BOM processes, though, lead to low productivity and costly errors.
With that in mind, why do so many manufacturers rely on substandard BOM management approaches like spreadsheets and email? Is there a better way for companies to support BOM-related processes?

Table of Contents
- BOM Processes Rely on Inadequate Solutions
- The BOM is the Fundamental Communication Tool
- The BOM Process Status Quo
- The Five Fundamentals of a Successful BOM Process
- 1) Aim for Accuracy
- 2) Keep Data Current
- 3) Manage Complete BOMs
- 4) Communicate BOM Data Clearly
- 5) Put BOM Data into Action
- Next Steps
- Acknowledgments
The BOM is the Fundamental Communication Tool
BOMs are the Backbone of the Manufacturing Industry BOMs are the fundamental way that manufacturers define, communicate, and realize their products. The bill of material is essentially the backbone of the manufacturing business, specifying what to buy and what to build. In many companies, BOM communication has to bridge disciplines across company boundaries. BOMs are The Primary Communication Tool in Industry Sound BOM processes create a bridge that crosses all parts of the organization. They document engineering designs and provide the information that Purchasing needs to order parts. Then, they deliver the data needed for Manufacturing to plan and execute orders. BOM data also serves as the backbone for others to roll up important attributes like weight, costs, and more. Successful BOM communication across Engineering, Purchasing and Manufacturing:
Successful BOM communication across Engineering, Purchasing and Manufacturing:
- Helps improve productivity
- Reduces the number of shipments delayed by missing parts
- Drives down scrap from using wrong parts or revisions
- Improves cost through item reuse
The BOM Process Status Quo
 Spreadsheets are the de facto Standard
The easiest way to sum up the BOM process status quo in most manufacturers is with a single word – spreadsheets. BOM processes frequently involve very complex spreadsheets. They are hard to interpret, lead to duplicating data, don’t manage data relationships, and often contain errors. As our BOM Management Buyer’s Guide shares, many companies manage BOMs in ways that don’t adequately support the business, including documents or embedding BOMs into CAD drawings in addition to spreadsheets.
Sharing BOMs via Email Creates Risk
Spreadsheets are not good for data management, and they are even more problematic when they’re shared through email. As soon as a BOM is attached to an email it creates a risk that somebody will access it after it is no longer valid. These informal methods are also challenging because data in a spreadsheet can’t easily be integrated with downstream people, processes, and systems. Because of this, unmanaged methods fall apart quickly for all but the simplest of companies.
Why Accept the Current Status Quo?
Why do manufacturers put up with this? Perhaps because it’s the way they’ve always done it. Perhaps they don’t recognize how many of their issues have poor BOM processes as the root cause. In many cases, they are just believe that fixing the problem is going to be complicated, time-consuming, and require expensive solutions.
They think that putting the basics of BOM management in place is going to slow them down, and that they’re not ready for a PLM or ERP system. This is particularly true for smaller companies. But big companies often run with immature BOM management processes as well. How can manufacturers upgrade the status quo without adding additional cost and overhead?
Spreadsheets are the de facto Standard
The easiest way to sum up the BOM process status quo in most manufacturers is with a single word – spreadsheets. BOM processes frequently involve very complex spreadsheets. They are hard to interpret, lead to duplicating data, don’t manage data relationships, and often contain errors. As our BOM Management Buyer’s Guide shares, many companies manage BOMs in ways that don’t adequately support the business, including documents or embedding BOMs into CAD drawings in addition to spreadsheets.
Sharing BOMs via Email Creates Risk
Spreadsheets are not good for data management, and they are even more problematic when they’re shared through email. As soon as a BOM is attached to an email it creates a risk that somebody will access it after it is no longer valid. These informal methods are also challenging because data in a spreadsheet can’t easily be integrated with downstream people, processes, and systems. Because of this, unmanaged methods fall apart quickly for all but the simplest of companies.
Why Accept the Current Status Quo?
Why do manufacturers put up with this? Perhaps because it’s the way they’ve always done it. Perhaps they don’t recognize how many of their issues have poor BOM processes as the root cause. In many cases, they are just believe that fixing the problem is going to be complicated, time-consuming, and require expensive solutions.
They think that putting the basics of BOM management in place is going to slow them down, and that they’re not ready for a PLM or ERP system. This is particularly true for smaller companies. But big companies often run with immature BOM management processes as well. How can manufacturers upgrade the status quo without adding additional cost and overhead?
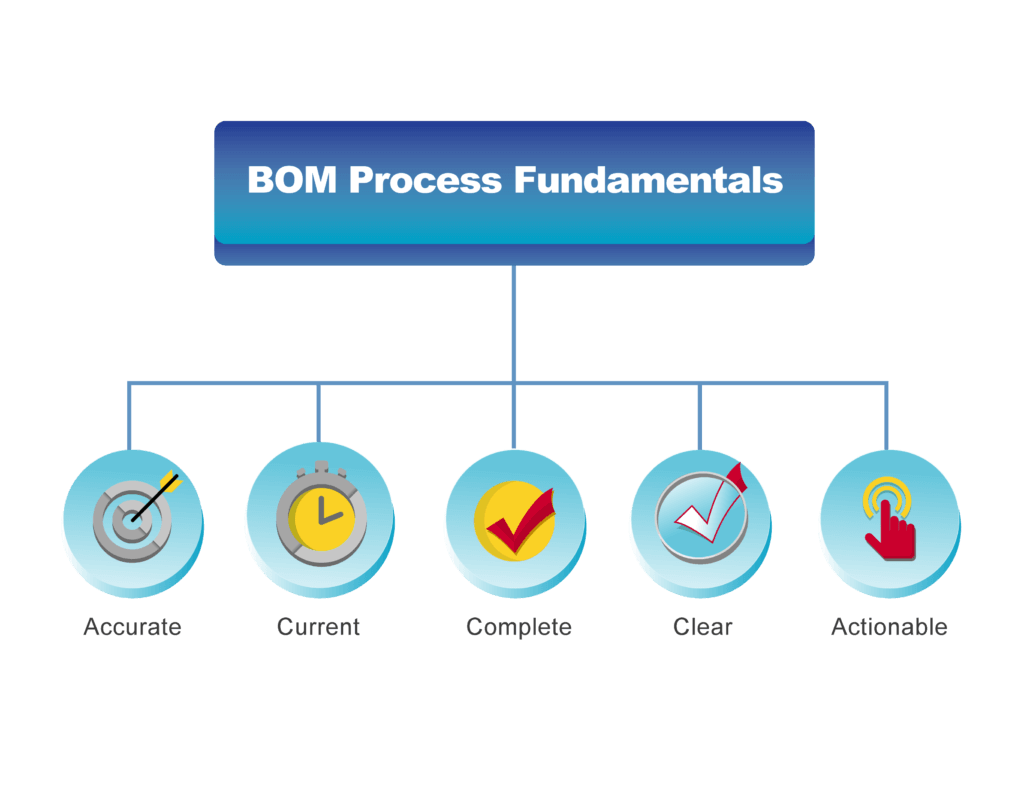
The Five Fundamentals of a Successful BOM Process
Get BOM Processes in Control Manufacturers who are ready to replace spreadsheets and email and get BOM processes under control can do so with reasonable effort. By focusing on the basics, they can make rapid improvements to productivity and reduce the impact of BOM-related errors. This leads to the question “What are the most important factors needed to improve BOM process?” We’ve identified five key areas that can easily be addressed and make a big impact on the business. Focus on the Fundamentals BOM Processes should be implemented and enabled in a way that ensures that they are:- Accurate
- Current
- Complete
- Clear
- Actionable
 Lower the Barriers and Increase Collaboration with a Targeted Cloud Solution
One option is using a targeted cloud solution built to address the five BOM Process Fundamentals. Many companies are considering cloud software because it lowers the barriers to adopting new technology. The cloud also helps ensure that everyone access the latest data, and that data is digitally accessible instead of locked up in files or on drawings. As David Anderson of Verdetech Products shares, “We’re trying to do everything on the web so we have universal data access.”
Lower the Barriers and Increase Collaboration with a Targeted Cloud Solution
One option is using a targeted cloud solution built to address the five BOM Process Fundamentals. Many companies are considering cloud software because it lowers the barriers to adopting new technology. The cloud also helps ensure that everyone access the latest data, and that data is digitally accessible instead of locked up in files or on drawings. As David Anderson of Verdetech Products shares, “We’re trying to do everything on the web so we have universal data access.”
1) Aim for Accuracy
 Provide Accurate Data Downstream
Manufacturers rely on their BOM to let downstream operations know what they need to execute on. Clearly, accurate information is important or the wrong parts could be ordered or an incorrect revision could be produced.
Get Data from the Source
How can companies make sure their information is accurate? First, existing data should be gathered directly from the source whenever possible instead of manually reentered. For BOM information, this means pulling product structures and metadata from CAD in order to capture the engineer’s intent. Beyond initially collecting this data, it must be kept in sync as designs change.
Allow Visibility and Collaboration
Secondly, the information should be available and visible to people across different roles, including those in the supply chain. They should have the ability to review and provide input based on their perspective and expertise.
Avoid Duplicate BOM and Item Information
Finally, information should have a single master source. This means that part information shouldn’t be input on a BOM by BOM basis. Part data should come from an item master that can be kept up to date centrally. Then, that data should be dynamically supplied to the BOM. This keeps information up to date, helps encourage accuracy, reduces duplication, and allows companies to determine where parts are used.
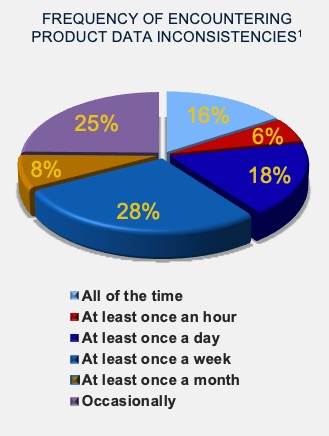
Unfortunately, our research shows that about two-thirds of companies find data inconsistencies between systems at least on a weekly basis. Part data duplication on each BOM will, by its nature, become inaccurate. This doesn’t have to be the case. “We have a parts catalog for parts used over and over in different projects to create a single version of truth,” says Michael White of Digitalcarbon. “I just edit my catalog and bring part data into my BOMs.”
Provide Accurate Data Downstream
Manufacturers rely on their BOM to let downstream operations know what they need to execute on. Clearly, accurate information is important or the wrong parts could be ordered or an incorrect revision could be produced.
Get Data from the Source
How can companies make sure their information is accurate? First, existing data should be gathered directly from the source whenever possible instead of manually reentered. For BOM information, this means pulling product structures and metadata from CAD in order to capture the engineer’s intent. Beyond initially collecting this data, it must be kept in sync as designs change.
Allow Visibility and Collaboration
Secondly, the information should be available and visible to people across different roles, including those in the supply chain. They should have the ability to review and provide input based on their perspective and expertise.
Avoid Duplicate BOM and Item Information
Finally, information should have a single master source. This means that part information shouldn’t be input on a BOM by BOM basis. Part data should come from an item master that can be kept up to date centrally. Then, that data should be dynamically supplied to the BOM. This keeps information up to date, helps encourage accuracy, reduces duplication, and allows companies to determine where parts are used.
Unfortunately, our research shows that about two-thirds of companies find data inconsistencies between systems at least on a weekly basis. Part data duplication on each BOM will, by its nature, become inaccurate. This doesn’t have to be the case. “We have a parts catalog for parts used over and over in different projects to create a single version of truth,” says Michael White of Digitalcarbon. “I just edit my catalog and bring part data into my BOMs.”
2) Keep Data Current
 Keep BOMs Up to Date
Outdated data has lost its value, regardless of how accurate it may have been. BOM data needs to be up to date, and manufacturers have to be confident that they can access the current version when they need it. How can manufacturers ensure they keep their BOMs current? In addition, how they can know what their BOM looked like at a certain point in time if they have to go back to resolve issues?
Put BOMs Under Change Control
BOM data has to be kept under a managed change process. Change management is critical to tracking revisions and history. It ensures that people accessing the BOM to do their job can be assured that they are working on the latest design. As Verdetech Products’ David Anderson shares, “When someone asks what the product costs, I don’t have to worry if it’s current or not.”
Avoid Duplicating BOM Data in Emails
Of course, data currency is highly dependent on how the data is shared and accessed. Information sent in a spreadsheet or document via email is immediately outdated. As soon as BOM data is attached to an email, it is no longer under control and creates a risk that someone will access outdated information to guide their work, leading to errors. “Once you copy a BOM and send it out by email you lose control if you make an adjustment or a change,” explains Michael White of Digitalcarbon.
Keep Data Centralized and Accessible
Email is a helpful tool for alerts and notifications, but not as a place to store data. Email alerts should point to common data. In many cases, companies are turning to the cloud because it’s accessible inside and outside of the business and everyone will always get the same.
Keep BOMs Up to Date
Outdated data has lost its value, regardless of how accurate it may have been. BOM data needs to be up to date, and manufacturers have to be confident that they can access the current version when they need it. How can manufacturers ensure they keep their BOMs current? In addition, how they can know what their BOM looked like at a certain point in time if they have to go back to resolve issues?
Put BOMs Under Change Control
BOM data has to be kept under a managed change process. Change management is critical to tracking revisions and history. It ensures that people accessing the BOM to do their job can be assured that they are working on the latest design. As Verdetech Products’ David Anderson shares, “When someone asks what the product costs, I don’t have to worry if it’s current or not.”
Avoid Duplicating BOM Data in Emails
Of course, data currency is highly dependent on how the data is shared and accessed. Information sent in a spreadsheet or document via email is immediately outdated. As soon as BOM data is attached to an email, it is no longer under control and creates a risk that someone will access outdated information to guide their work, leading to errors. “Once you copy a BOM and send it out by email you lose control if you make an adjustment or a change,” explains Michael White of Digitalcarbon.
Keep Data Centralized and Accessible
Email is a helpful tool for alerts and notifications, but not as a place to store data. Email alerts should point to common data. In many cases, companies are turning to the cloud because it’s accessible inside and outside of the business and everyone will always get the same.
3) Manage Complete BOMs
 Create Complete Product Definitions
Manufacturers rely on their BOMs to communicate the complete product composition, and do it in the right context. A BOM isn’t just a collection of parts or a CAD structure. While CAD integration is important, it doesn’t tell the whole story. As David Anderson of Verdetech Products explains, the BOM can communicate a rich amount of product data. “There’s a mass of information that’s all interrelated, it’s important to encapsulate that knowledge instead of going back through emails and trying to recreate it,” he says.
Manage BOM Relationships to Prevent Data Duplication
This information can include technical specifications like materials or commercial information like preferred suppliers. As mentioned earlier, it’s important not to duplicate information from the item master. In the same way, vendor information should come from a vendor database so the information can be validated, there’s no duplication, and Purchasing can find all references where the vendor is used.
Organize BOM Data by Role
BOM structures don’t always fit the right format for execution, and have to be modified to provide the right information. For example, companies may want to add non-modeled parts, consumables, tooling, or other information. Also, people have different perspectives and need different data to play their role. For example, a designer might model an assembly with all of its underlying parts, but Purchasing my view it as a single item from a supplier. In a similar way, third parties like suppliers may only require access rights to a subset of information. Data has to be organized for the intended user and match their execution needs.
Create New Knowledge
BOM data can be used to calculate new details, including cost rollups, weights, ordered quantities, and more. These calculations are very hard to accomplish in spreadsheets due to the way they are organized. BOM data can also provide a rich source of insight by applying business intelligence and machine learning. The key consideration, though, is that all of this information should be kept in context.
Create Complete Product Definitions
Manufacturers rely on their BOMs to communicate the complete product composition, and do it in the right context. A BOM isn’t just a collection of parts or a CAD structure. While CAD integration is important, it doesn’t tell the whole story. As David Anderson of Verdetech Products explains, the BOM can communicate a rich amount of product data. “There’s a mass of information that’s all interrelated, it’s important to encapsulate that knowledge instead of going back through emails and trying to recreate it,” he says.
Manage BOM Relationships to Prevent Data Duplication
This information can include technical specifications like materials or commercial information like preferred suppliers. As mentioned earlier, it’s important not to duplicate information from the item master. In the same way, vendor information should come from a vendor database so the information can be validated, there’s no duplication, and Purchasing can find all references where the vendor is used.
Organize BOM Data by Role
BOM structures don’t always fit the right format for execution, and have to be modified to provide the right information. For example, companies may want to add non-modeled parts, consumables, tooling, or other information. Also, people have different perspectives and need different data to play their role. For example, a designer might model an assembly with all of its underlying parts, but Purchasing my view it as a single item from a supplier. In a similar way, third parties like suppliers may only require access rights to a subset of information. Data has to be organized for the intended user and match their execution needs.
Create New Knowledge
BOM data can be used to calculate new details, including cost rollups, weights, ordered quantities, and more. These calculations are very hard to accomplish in spreadsheets due to the way they are organized. BOM data can also provide a rich source of insight by applying business intelligence and machine learning. The key consideration, though, is that all of this information should be kept in context.
4) Communicate BOM Data Clearly

 Focus on Clarity of Communication
A BOM isn’t valuable unless it can be easily understood by the people that need product information to do their jobs. Remember, it’s a communication tool. Too frequently, BOMs are large lists of numbers that mean very little to most people. How can companies ensure their BOMs are usable by others?
Create Personalized Data Views
First, different people need different views to make the BOM clear. It’s import to provide information that’s role and task appropriate. For example, people in the supply chain don’t know your part numbers and you probably only want to share a subset of information with them.
Present BOMs in a Visual Context
Second, information should be presented in a visual, intuitive way. Thumbnails of parts, particularly if they are 3D, are much more approachable than long lists of numbers. Multilevel views that allow you to expand, contract, flatten, and drill down to get more information or access CAD files are more valuable than complex colors and indentations in spreadsheets.
Make BOM Data Accessible Downstream Digitally
Lastly, data needs to be interpreted by other systems as well as people. BOM data should be accessible in a database, not locked up in a spreadsheet or CAD file.
Focus on Clarity of Communication
A BOM isn’t valuable unless it can be easily understood by the people that need product information to do their jobs. Remember, it’s a communication tool. Too frequently, BOMs are large lists of numbers that mean very little to most people. How can companies ensure their BOMs are usable by others?
Create Personalized Data Views
First, different people need different views to make the BOM clear. It’s import to provide information that’s role and task appropriate. For example, people in the supply chain don’t know your part numbers and you probably only want to share a subset of information with them.
Present BOMs in a Visual Context
Second, information should be presented in a visual, intuitive way. Thumbnails of parts, particularly if they are 3D, are much more approachable than long lists of numbers. Multilevel views that allow you to expand, contract, flatten, and drill down to get more information or access CAD files are more valuable than complex colors and indentations in spreadsheets.
Make BOM Data Accessible Downstream Digitally
Lastly, data needs to be interpreted by other systems as well as people. BOM data should be accessible in a database, not locked up in a spreadsheet or CAD file.
5) Put BOM Data into Action
 Put BOMs to Use
Information is important, but has to be put to use to provide value. How can manufacturers ensure that their data can be used to drive higher efficiency and reduce errors?
Extend BOM Knowledge to Support Downstream Processes
Effective BOM execution combines data with BOM processes. It creates an integrated process that connects design intent from Engineering with what’s needed to fulfill it downstream. BOM quantities aren’t for reference only, they should be accessible for procurement requirements and manufacturing quantity calculations. They should also be available for rollups like costs or material quantities to make decisions.
Leverage BOM Explosions for Procurement and Production Planning
A BOM solution should also allow BOM explosions to help plan production and communicate demand to suppliers. It should share that information with an ERP system if available.
Consider Simpler Solutions
Some solutions are blurring the lines between BOM definition and execution by supporting additional BOM-related tasks like orders and. These solutions are worth a look, can provide value with fewer barriers to adoption, and can serve as a foundation to grow from at the point the company is ready for more formal systems like PDM, PLM, and/or ERP.
Put BOMs to Use
Information is important, but has to be put to use to provide value. How can manufacturers ensure that their data can be used to drive higher efficiency and reduce errors?
Extend BOM Knowledge to Support Downstream Processes
Effective BOM execution combines data with BOM processes. It creates an integrated process that connects design intent from Engineering with what’s needed to fulfill it downstream. BOM quantities aren’t for reference only, they should be accessible for procurement requirements and manufacturing quantity calculations. They should also be available for rollups like costs or material quantities to make decisions.
Leverage BOM Explosions for Procurement and Production Planning
A BOM solution should also allow BOM explosions to help plan production and communicate demand to suppliers. It should share that information with an ERP system if available.
Consider Simpler Solutions
Some solutions are blurring the lines between BOM definition and execution by supporting additional BOM-related tasks like orders and. These solutions are worth a look, can provide value with fewer barriers to adoption, and can serve as a foundation to grow from at the point the company is ready for more formal systems like PDM, PLM, and/or ERP.
Next Steps
Go Beyond the Status Quo to Prevent Mistakes Today’s BOM process status quo, frequently relying on inadequate technology like spreadsheets and email, leads to inefficiency, excess cost, mistakes, quality issues, and late orders. The consequences of poor processes, especially for a smaller company, can be significant. As David Anderson of Verdetech Products shares, “Sometimes you get away with things by luck, but a major problem could kill a company.” It’s time for companies to raise the bar on BOM data and processes. Take Advantage of Performance Improvements
Effective BOM management creates a cohesive process that connects everything from design to purchase orders to production. It goes further to provide the right information, including historical data, to resolve service issues. An effective process leverages a wealth of information in context, including CAD, items, and vendors, without duplicating data or allowing it to be shared without control.
Take Advantage of Performance Improvements
Effective BOM management creates a cohesive process that connects everything from design to purchase orders to production. It goes further to provide the right information, including historical data, to resolve service issues. An effective process leverages a wealth of information in context, including CAD, items, and vendors, without duplicating data or allowing it to be shared without control.
 Get Started
For many companies, implementing traditional systems may feel too cumbersome. But that doesn’t mean they can’t improve. Manufacturers can focus on the five identified opportunities to improve BOM processes and improve performance by using lighter weight, targeted cloud solutions. Cloud software fosters collaboration and comes with lower barriers to adoption. These systems can help manufacturers take a big step in the right direction and provide room to grow in the future. It’s time to get started, start small, and incrementally improve BOM processes to reduce errors and improve performance.
Get Started
For many companies, implementing traditional systems may feel too cumbersome. But that doesn’t mean they can’t improve. Manufacturers can focus on the five identified opportunities to improve BOM processes and improve performance by using lighter weight, targeted cloud solutions. Cloud software fosters collaboration and comes with lower barriers to adoption. These systems can help manufacturers take a big step in the right direction and provide room to grow in the future. It’s time to get started, start small, and incrementally improve BOM processes to reduce errors and improve performance.
Acknowledgments
About the Author Jim is a recognized expert in enterprise software for manufacturers with over 25 years of experience in application software, management consulting, and research. He has extensive knowledge on how industrial companies use product innovation, product development, engineering, and other enterprise solutions to improve business performance. Jim is actively researching the value of improving product innovation and operational performance through digitalization. [post_title] => The Five Basics of Effective BOM Processes (eBook) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => billofmaterials-management [to_ping] => [pinged] => [post_modified] => 2022-11-14 22:28:16 [post_modified_gmt] => 2022-11-15 03:28:16 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7823 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 1 [filter] => raw ) [4] => WP_Post Object ( [ID] => 7799 [post_author] => 2572 [post_date] => 2019-05-01 14:32:36 [post_date_gmt] => 2019-05-01 18:32:36 [post_content] =>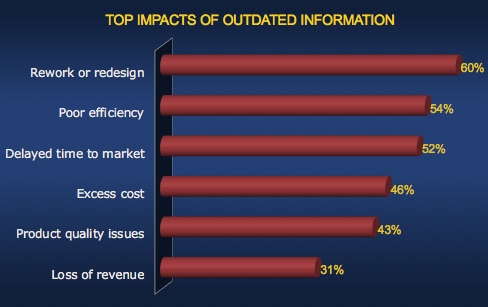 Imagine what you could do if you could get almost a third of your time back? In a guest post on the Sodius Willert blog, Michelle Boucher shares research on how much engineering time is wasted on non-value added work and explains what you can do.
Imagine what you could do if you could get almost a third of your time back? In a guest post on the Sodius Willert blog, Michelle Boucher shares research on how much engineering time is wasted on non-value added work and explains what you can do.
Focus on the Work You Enjoy
As an engineer, what gets you excited about your job? For most, it’s getting to work on really cool projects and innovating. Unfortunately, a good chunk of your time is wasted on other things, taking you away from the work you enjoy doing and the work that adds value for your company.How Do You Limit Non-Value Added Work?
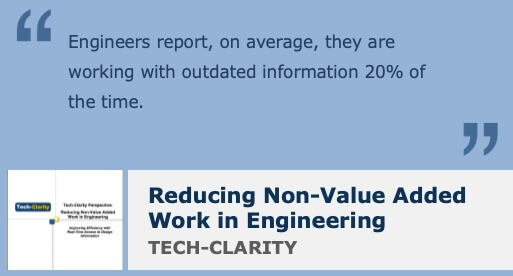
 Research from Tech-Clarity’s Reducing Non-Value Added Work in Engineering reveals that engineers waste a startling 32% on non-value added work. That’s a lot of lost opportunity. Imagine what you could do if you could get almost a third of your time back? Much of this non-value added work is related to the many manual tasks associated with managing, sharing, and finding data. Data tends to be stored in multiple places, so there is no single source of truth.
Without a single source of truth, there is no traceability across the data. It then becomes practically impossible to identify the impact of an update to requirements, new design work, or changes to other parts of the design. Without traceability, changes can not automatically propagate across all product data. Instead, changes and updates become a very manual, if not impossible, process to identify everything that is impacted and update it. This is further complicated if the same data is located in multiple places. The situation creates a significant risk for outdated and conflicting data. Unfortunately, this costs the company in numerous ways, which we will explore further.
Research from Tech-Clarity’s Reducing Non-Value Added Work in Engineering reveals that engineers waste a startling 32% on non-value added work. That’s a lot of lost opportunity. Imagine what you could do if you could get almost a third of your time back? Much of this non-value added work is related to the many manual tasks associated with managing, sharing, and finding data. Data tends to be stored in multiple places, so there is no single source of truth.
Without a single source of truth, there is no traceability across the data. It then becomes practically impossible to identify the impact of an update to requirements, new design work, or changes to other parts of the design. Without traceability, changes can not automatically propagate across all product data. Instead, changes and updates become a very manual, if not impossible, process to identify everything that is impacted and update it. This is further complicated if the same data is located in multiple places. The situation creates a significant risk for outdated and conflicting data. Unfortunately, this costs the company in numerous ways, which we will explore further.
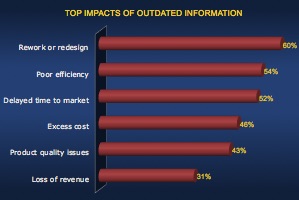
The Cost of Outdated Information
When design work is based on outdated information, error will be inevitable. These errors result in costly rework and redesign. Wasting even more time to correct these problems means poor efficiency which can delay time to market. As the design nears completion, there are fewer options to fix problems, so errors become much more expensive and time consuming to fix. Plus, with limited options, solutions may be less than ideal, which will hurt product quality or drive up cost. Of course, if problems are missed entirely, there will be even bigger quality problems in the field. The combination of poor quality, higher cost, and late to market all hurt competitiveness which can reduce revenue opportunities. This problem gets even worse when multiple engineering disciplines are involved. When products integrate mechanical components, electronics, and embedded software, there are inherent silos. Continue to the Sodius Willert blog for the full post. [post_title] => How to Avoid Non-Value Added Work for Engineers (guest post) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => non-value-added-work [to_ping] => [pinged] => [post_modified] => 2022-11-14 22:25:36 [post_modified_gmt] => 2022-11-15 03:25:36 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7799 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [5] => WP_Post Object ( [ID] => 7516 [post_author] => 2572 [post_date] => 2019-04-25 11:30:48 [post_date_gmt] => 2019-04-25 15:30:48 [post_content] =>How do you navigate complex, omnichannel environments and manage multiple sources of data to make the best decisions? Can predictive analytics help?
Tech-Clarity’s Retail Analytics Buyer's Guide explains how predictive analytics provide better visibility across your retail business so that you can make better decisions to become more competitive. As the retail industry faces significant disruption, new approaches are required to survive. With volatile markets and fierce competition, the right decisions are critical to survival. Unfortunately, complex omnichannel environments, global supply chains, and dynamic development teams make it practically impossible to get timely visibility into the business. Even if you can, will you have confidence in your decisions? With the right predicative analytics solution, you can overcome these challenges, but only if you select the right technology. The research identifies eight capabilities to look for in a predicative analytics solution. These capabilities will help you make better decisions about your retail business.
Click here for the full eBook, thank you to our sponsor PTC.
WHY ARE PREDICTIVE ANALYTICS CRITICAL FOR TODAY’S RETAILERS?
In the retail industry, you need to support a complex, global, omnichannel environment, while navigating a dynamic market filled with economic volatility. It is not easy, especially as consumers have become more empowered and demanding through social media and online resources. To be successful, you need to make fast decisions, identify opportunities, respond to changing trends, react to competitors, adjust to shifts in the supply chain, and more. It’s complex. In many cases, margins are so thin that missing a trend, failing to spot an opportunity, or making the wrong decision can significantly hurt profitability. A few weak seasons can be disastrous for a brand.

Making things even harder, the barriers to entry have dropped. It’s no longer enough to compete by becoming the biggest brand and leveraging scale. Online marketplaces have expanded the reach of start-ups and small companies. In many cases, the playing field has been leveled as resources that were only available to the largest companies are now available to everyone. This situation has further intensified the competition. Retail companies who can respond to market changes before their competitors will have the advantage.
To survive, retail companies must be nimble enough to react quickly, but they can only do that with the right insights. Information and knowledge are now competitive weapons and technology can be key to unleashing its potential. That knowledge comes from every aspect of the organization from business operations, product trends, supplier information, and more. This buyer’s guide reveals how retail companies can capitalize on their knowledge and uncovers eight capabilities that will help put the right technology in place to support them.
HOW DO PREDICTIVE ANALYTICS HELP?
The top challenges uncovered by research conducted by BOF and McKinsey reveal the importance of getting instant insights. The economic uncertainty related to events such as Brexit, a rise in protectionist policies in the US, a volatile stock market, and more can erode consumer confidence and make it even harder to predict buying trends. Key insights will help overcome some of this uncertainty. With online shopping and social media, consumers are more informed than ever, creating a need for retailers to know how they can best influence buying behavior. The right data, in context, will help. Merchandise needs to be at the right place at the right time while avoiding excess inventory. Retailers need to offer consumers the experiences that will keep them coming back, with the right products that reflect the latest trends. Again, analytics will guide those decisions.
SELECT THE RIGHT RETAIL PREDICTIVE ANALYTICS SOLUTION FOR YOUR NEEDS
By selecting the right software, you should get timely insights to help you bring the right products to market, optimize the management of supply chains, influence buying behavior and more. These needed insights will help you make smarter decisions which should lead to better operating margins
*This summary is an abbreviated version of the eBook and does not contain the full content. A link to download the full eBook is available above.
If you have difficulty obtaining a copy of the research, please contact us using the “Contact” link below.
[post_title] => Retail Analytics Buyer’s Guide (eBook) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => retail-analytics-buyers-guide [to_ping] => [pinged] => [post_modified] => 2022-11-14 22:28:15 [post_modified_gmt] => 2022-11-15 03:28:15 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7516 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [6] => WP_Post Object ( [ID] => 7737 [post_author] => 2572 [post_date] => 2019-04-22 12:21:38 [post_date_gmt] => 2019-04-22 16:21:38 [post_content] => As our changing world impacts product design, how should CAD evolve? How can CAD support your efforts to adopt some of the latest technologies?
During the PTC on-demand virtual session, “The Renaissance of CAD: What's New, What's Now & What You Can Do With It” you will hear about some of the latest advancements in product design and how CAD has evolved to support them. Sessions include topics on IoT, generative design, simulation, additive manufacturing, and augmented reality.
During the simulation session, Tech-Clarity’s Michelle Boucher shares highlights from her recent research on simulation and how CAE can be a valuable tool for design engineers. Watch Michelle's session here. To read more about Michelle's research on simulation click here.
Watch all the sessions here.
As our changing world impacts product design, how should CAD evolve? How can CAD support your efforts to adopt some of the latest technologies?
During the PTC on-demand virtual session, “The Renaissance of CAD: What's New, What's Now & What You Can Do With It” you will hear about some of the latest advancements in product design and how CAD has evolved to support them. Sessions include topics on IoT, generative design, simulation, additive manufacturing, and augmented reality.
During the simulation session, Tech-Clarity’s Michelle Boucher shares highlights from her recent research on simulation and how CAE can be a valuable tool for design engineers. Watch Michelle's session here. To read more about Michelle's research on simulation click here.
Watch all the sessions here.
 [post_title] => The Renaissance of CAD
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cad-renaissance-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-20 00:28:44
[post_modified_gmt] => 2024-01-20 05:28:44
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7737
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[7] => WP_Post Object
(
[ID] => 7752
[post_author] => 2
[post_date] => 2019-04-15 08:41:20
[post_date_gmt] => 2019-04-15 12:41:20
[post_content] => How can food and beverage companies use digitalization to dramatically increase flexibility, enhance consumer responsiveness, and improve productivity? This episode of Tech-Clarity TV, Revolutionizing Food and Beverage Production with the Industrial IoT and the Digital Twin, shares how food producers can leverage digital twins and the IoT to improve performance.
https://youtu.be/kGb6utklQwk
[post_title] => The Renaissance of CAD
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cad-renaissance-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-20 00:28:44
[post_modified_gmt] => 2024-01-20 05:28:44
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7737
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[7] => WP_Post Object
(
[ID] => 7752
[post_author] => 2
[post_date] => 2019-04-15 08:41:20
[post_date_gmt] => 2019-04-15 12:41:20
[post_content] => How can food and beverage companies use digitalization to dramatically increase flexibility, enhance consumer responsiveness, and improve productivity? This episode of Tech-Clarity TV, Revolutionizing Food and Beverage Production with the Industrial IoT and the Digital Twin, shares how food producers can leverage digital twins and the IoT to improve performance.
https://youtu.be/kGb6utklQwk
Transcript
The food and beverage industry is facing major disruption New competitors are adopting digital technologies and taking on the largest of companies, competing with unprecedented innovation and agility. Consumers are demanding more. To rise to the challenge, food and beverage plants need to increase flexibility while maintaining high productivity and compliance to ensure consumer confidence. The digital revolution has begun. Food companies have to leverage digital tools including the Internet of Things, the Digital Twin, the Cloud and Analytics to drive agility and consumer responsiveness. Using the IoT, digital food and beverage companies tap into data from a variety of sources and use analytics to gain deep consumer insights. Then, they quickly reformulate digital recipes to respond to trends. They leverage plant automation to capitalize on changing preferences. Digitalization allows food and beverage companies to get products that consumers want to market faster than the competition. But leading companies go beyond just internal agility and responsiveness. They use the IoT to gain advanced insights to optimize recipes and production schedules. The digital plant acts as one with their supply chain to rapidly adjust to changing supply and demand. Digital food and beverage companies also turn to digitalization to drive higher productivity. They reduce downtime by monitoring equipment health and servicing equipment before it fails using the Industrial IoT and predictive maintenance. They improve yields by identifying and correcting production issues in real-time. They use the IoT and analytics to benchmark performance to identity improvement opportunities. They use the digital twin of their plant to simulate and validate the impact of changing equipment. They quickly deploy new recipes, packaging and equipment using virtual commissioning. Finally, they identify opportunities to more accurately simulate production based on IoT data and a closed loop digital twin to better reflect reality and improve optimization using real-world information. Digitalization helps food and beverage companies improve throughput, increase uptime, decrease cost, improve quality and support high levels of traceability and compliance. It enables companies to raise the bar on innovation and agility without compromising productivity, cost or quality. The time has come for food and beverage plants to digitalize in order to survive in the digital age. To learn how, watch the rest of our video series on digitalization in the food & beverage industry. And learn more from our sponsor, Siemens PLM, a leader in digitalization for the food & beverage industry. [post_title] => IoT, Industrial IoT, and Digital Twins in Food & Beverage Production (animation) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => iot-twin-foodbeverage [to_ping] => [pinged] => [post_modified] => 2022-12-02 15:05:38 [post_modified_gmt] => 2022-12-02 20:05:38 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7752 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [8] => WP_Post Object ( [ID] => 7580 [post_author] => 2572 [post_date] => 2019-04-03 14:25:01 [post_date_gmt] => 2019-04-03 18:25:01 [post_content] =>How can you get the quickest return on your investments in technology?
As we look ahead to the coming decade, technology will become increasingly critical for your company to stay competitive. New technology can include CAD, CAE, PLM, 3D printing, IoT, and more. However, for that technology to work for your business, your implementation must be successful. With a new technology, often it is the cultural changes that can derail the project. Starting with a plan to overcome challenges like this can be the most important part of a successful implementation. Tech-Clarity’s Ten Practices to Successfully Implement Technology shares 10 best practices to support the implementation and adoption of new technology so that you can get the expected return on your technology investments as quickly as possible.
Please enjoy the summary* below.
For the full eBook, please visit our sponsor SOLIDWORKS (free of charge, registration required).
HOW CAN YOU GET THE MOST FROM YOUR TECHNOLOGY INVESTMENTS?
Staying competitive in today’s global market means developing exceptional products that are innovative, high quality, and cost-effective. With so much to consider, how can you get ahead? For many, technology, including software tools, is the answer. Technology investments can help you grow your business, expand your customer base, and extend your services. With great software tools, you can accomplish more than you thought possible. Unfortunately, ignoring opportunities for improvement can stagnate the business, which can eventually lead to lost market share.
The key is selecting the right technology solution and successfully adopting it. This eBook identifies best practices for successful technology adoption to position your company for success for the next decade.
TECHNOLOGY SUPPORTS INNOVATION EFFORTS
Outsource vs. Internal Development?
Innovation often requires new skills. Many find they lack the internal expertise to take advantage of new materials, use the latest manufacturing techniques, support connectivity, or benefit from other industry advancements. When faced with this challenge, you can outsource or try to develop the skills internally. Regardless of which option you choose, technology will make it easier.
Support Outsourcing with Technology
For some, outsourcing can be a great option as a way to tap into expertise that you lack. When outsourcing, technology can ensure good collaboration, support communication, keep design data in sync, and protect intellectual property. As an example, cloud solutions can offer a platform to share data with third parties in a secure manner.
Technology Lowers Internal Development Cost
For other companies, especially smaller businesses, the overhead associated with outsourcing may be too much. It takes time to familiarize a third party with your product lines, manage quality, and coordinate changes, especially for highly engineered products. In these cases, it may be easier to do everything in-house. Again, technology can make that easier with software tools that have embedded intelligence to guide the design or manufacturing process.
RECOMMENDATIONS AND NEXT STEPS
Technology can have a significantly impact on your business in a positive way. It can be the key to help you expand, grow, and evolve your business. However, as good as the technology may be, it will only help your company if you take the right steps to ensure the adoption is successful.
The following checklist will help you achieve success:

- Determine Your Business Needs Upfront
- Be Open to Change
- Keep the Focus on Customers
- Consider Vision and Future Needs
- Keep it Simple
- Automate Tedious Workflows
- Manage Scope
- Manage the Role of Team Members
- Overcome the Resistance to Change
- Provide Training
- Establish Success Factors
By following these ten recommendations, your company will be better positioned to successfully adopt technology and realize the expected ROI even faster.
*This summary is an abbreviated version of the eBook and does not contain the full content. A link to download the full eBook is available above.
If you have difficulty obtaining a copy of the research, please contact us using the “Contact” link below.
[post_title] => Ten Practices to Successfully Implement Technology (eBook) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => ten-practices-to-successfully-implement-technology-ebook [to_ping] => [pinged] => [post_modified] => 2022-11-14 22:28:16 [post_modified_gmt] => 2022-11-15 03:28:16 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7580 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [9] => WP_Post Object ( [ID] => 7670 [post_author] => 2 [post_date] => 2019-04-01 09:44:21 [post_date_gmt] => 2019-04-01 13:44:21 [post_content] => How have manufacturers' opinions on using the cloud to support product innovation, product development, and engineering changed? How does that impact cloud adoption? Read the guest post in full in the Digital Transformation section of the Siemens PLM Community blog. Jim Brown recently shared a guest post on the Siemens' blog offering our experience on cloud adoption and starts a discussion about the variety of benefits that engineering software on the cloud offers. The post includes information from our prior research on using cloud for PLM, including this flowchart for companies to use when considering their cloud strategy.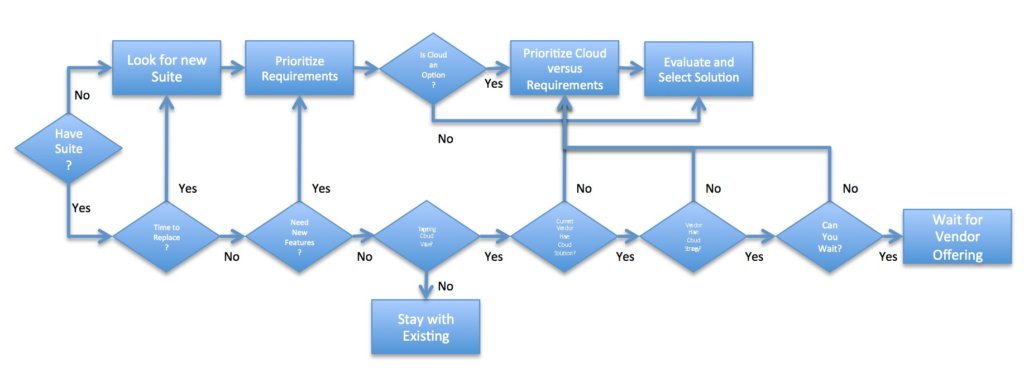 Times have changed. We've seen a shift from companies questioning if they should use the cloud to asking "why not?" use the cloud. Many viewed slower cloud adoption for PLM and other engineering solutions as a reluctance to put IP in the cloud, but our research and experience point to another reason. The solutions available on the cloud versus more traditional deployments simply did not offer equivalent capabilities. Now, we're seeing a different mindset.
Our conclusion is that the appetite for cloud solutions is increasing. At the same time, options are expanding to include more traditional solutions, along with their deep functionality, in a cloud offering. It’s time for more companies to take a look.
[post_title] => Why Not Adopt Cloud for Product Innovation and Engineering? (guest post)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-adoption
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:36
[post_modified_gmt] => 2022-11-15 03:25:36
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7670
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[10] => WP_Post Object
(
[ID] => 7590
[post_author] => 2572
[post_date] => 2019-03-26 23:07:40
[post_date_gmt] => 2019-03-27 03:07:40
[post_content] =>
Times have changed. We've seen a shift from companies questioning if they should use the cloud to asking "why not?" use the cloud. Many viewed slower cloud adoption for PLM and other engineering solutions as a reluctance to put IP in the cloud, but our research and experience point to another reason. The solutions available on the cloud versus more traditional deployments simply did not offer equivalent capabilities. Now, we're seeing a different mindset.
Our conclusion is that the appetite for cloud solutions is increasing. At the same time, options are expanding to include more traditional solutions, along with their deep functionality, in a cloud offering. It’s time for more companies to take a look.
[post_title] => Why Not Adopt Cloud for Product Innovation and Engineering? (guest post)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-adoption
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:36
[post_modified_gmt] => 2022-11-15 03:25:36
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7670
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[10] => WP_Post Object
(
[ID] => 7590
[post_author] => 2572
[post_date] => 2019-03-26 23:07:40
[post_date_gmt] => 2019-03-27 03:07:40
[post_content] =>
How do you empower engineers to design the best products possible?
Research from Tech-Clarity’s Revolutionizing Simulation For Design Engineers research report finds that design engineers lack confidence in design decisions 28% of the time. The research identifies the most common ways engineers deal with this uncertainty, its impact, and how to improve confidence. This research study, based on a survey of 195 companies, examines the design process and identifies top challenges that hold engineers back. The report reveals how to empower engineers with insight to improve product quality, lower cost, and accelerate time-to-market, all while developing more innovative products and the role that CAE can play.
Please enjoy the summary* below.
For the full research report, please visit our sponsor PTC (free of charge, registration required).
ENGINEERING DECISIONS CAN MAKE OR BREAK YOUR PRODUCTS
How do you empower engineers to design the best products possible?
Engineers want to design great products. Unfortunately, factors like increasing product complexity, competing design criteria, and knowing how design decisions impact other parts of the design make it hard. On top of this, ever-shrinking timelines mean engineers have their work cut out for them. Yet, exceptional engineering has become critical to success in today’s competitive global market.
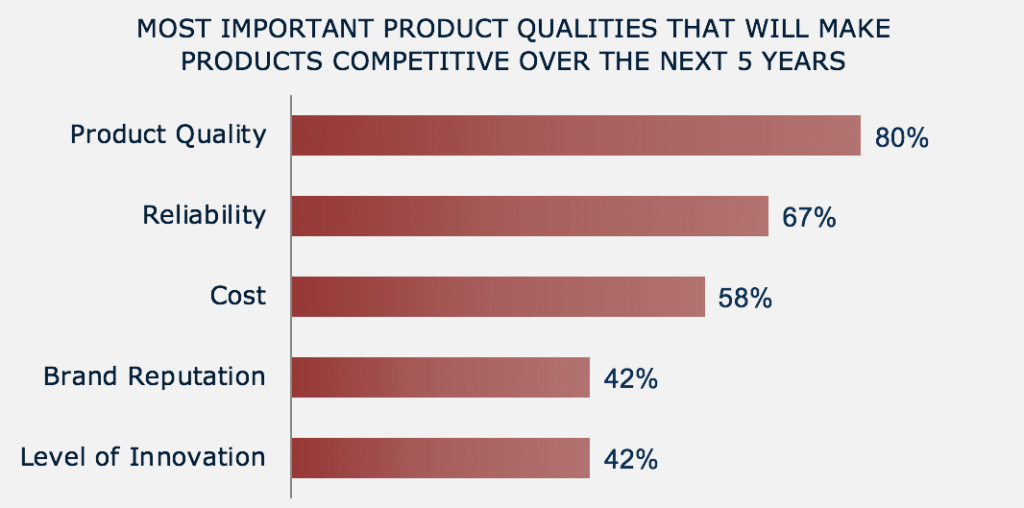
WHAT’S MOST IMPORTANT FOR YOUR PRODUCT’S MARKET SUCCESS?
Product Quality
As companies face mounting pressure from global competitors, engineering criteria have become essential to competitively differentiate products. In fact, 80% of survey respondents believe that product quality is the most important product attribute to keep products competitive (see graph). Reliability and cost come next. This indicates customers have high expectations for quality and durability but don’t want to overpay. To be successful, companies should balance these criteria.
Engineering Decisions Are Critical – and Not Easy
Requirements for quality, reliability, and cost often conflict so balancing them is no small feat. Unfortunately, product complexity makes it hard for engineers to know the full impact of each design decision. Indeed, 76% of survey respondents rate design decisions that impact product competitiveness as ‘somewhat hard’ to ‘extremely difficult.’ This leads many engineers to overengineer, which unfortunately drives up cost.
Companies who can make this decision process easier will have an advantage.
RECOMMENDATIONS TO IMPROVE ENGINEERING DECISIONS
To help improve engineering decisions, Tech-Clarity offers the following recommendations:
- Empower design engineers with simulation tools to help guide their decisions.
- Use simulation as early as possible during concept and design.
- Rely on simulation as a design tool to optimize the design and provide directional guidance.
- Consider new approaches to simulation that are tailored for design engineers and make setting up an analysis easier.
- Look for a simulation solution that can offer instant results.
*This summary is an abbreviated version of the report and does not contain the full content. A link to download the full report is available above.
If you have difficulty obtaining a copy of the research, please contact us using the “Contact” link below.
[post_title] => Revolutionizing Simulation For Design Engineers [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => revolutionizing-simulation-design-engineers [to_ping] => [pinged] => [post_modified] => 2024-01-20 00:23:15 [post_modified_gmt] => 2024-01-20 05:23:15 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7590 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [11] => WP_Post Object ( [ID] => 7596 [post_author] => 2 [post_date] => 2019-03-26 11:32:12 [post_date_gmt] => 2019-03-26 15:32:12 [post_content] =>
What should Life Sciences companies look for in a solution to help them simultaneously improve profitability and patient outcomes using remote device monitoring via the IoT?
Our new Improving Service Performance and Patient Outcomes with Remote Monitoring Buyer's Guide helps Life Sciences companies understand the business and social value of monitoring equipment with the IoT. It then offers evaluation criteria for companies to use as requirements to select a solution to achieve that value. The research also shares the experience of Varian Medical Systems and how they've used the IoT to drive improved service and customer relationships.
Please enjoy the summary below. For the full report, please visit our sponsor PTC (no charge, registration required).
Life Sciences Companies are Dramatically Improving Service by Digitalizing
Digitalization is changing the way innovative medical device, biotech, and related companies provide value and drive profitability. Research shows that one of the most compelling digital transformation opportunities is to revolutionize service, specifically:
- Deloitte finds1 that predictive maintenance can increase uptime and availability by 10-20 percent and reduce overall maintenance costs 5-10 percent
- Tech-Clarity’s Michelle Boucher shares2 that service is critical, that medical device downtime can impact lives, and that predictive maintenance can offer a significant advantage to minimize patient impact
Table of Contents
- Improve Service ROI, Build your IoT Foundation for the Future
- Remote Monitoring Drives Service Performance
- Access Equipment and Device Data
- Communicate with Devices
- Leverage the Edge to Pre-Process Data
- Share Actionable Service Information
- Plan for Implementation and Adoption
- Select the Right Strategic Partner
- Next Steps
- Buyer’s Guide Checklist
- Acknowledgments
Improve Service ROI, Build your IoT Foundation for the Future
Industries are Digitally Transforming
Life Sciences companies have a tremendous opportunity to improve service operations and outcomes. At the same time, many are investigating how to benefit from the Internet of Things (IoT). Remotely monitoring medical equipment is a great way to accomplish both.
As our related Improving Service with Remote Monitoring Buyer’s Guide explains, a remote monitoring initiative allows companies to quickly achieve IoT value. In fact, the most common way that companies gain tangible ROI from IoT is through improved service. At the same time, it also paves the way for even more substantial benefits from initiatives like healthcare analytics.
Equipment Monitoring Has Proven Value
The IoT lets companies transform service to generate more – and more profitable – service revenue. They do this by moving from reactive to proactive to predictive service, and leveraging advanced technologies like AI, machine learning, and big data analytics. They can also adopt new service delivery processes like remote service. But the most common first step is reducing cost of service through remote monitoring.
Next Steps
Get Started
Leveraging the IoT can help companies improve service for themselves and their customers by reducing cost and transitioning to proactive and predictive service. Remote monitoring allows companies to identify and resolve issues remotely, providing faster service and increased uptime while reducing the cost of truck rolls and putting service technicians on site.

It can go beyond cost savings to create a new source of revenue from paid upgrades or remotely enhancing equipment capabilities or permissions by “unlocking” enhanced capabilities via a subscription. It also offers Life Sciences companies an opportunity to help ensure devices are available and operating optimally to serve patient needs.
Improve Service and Patient Outcomes
Medical device, biotech and other life science innovators embarking on a remote monitoring initiative to improve service should look for the capabilities outlined in the sections of this guide to help ensure they get the value they seek. In addition, they can use the following checklist as high-level criteria to compare offerings.
*This summary is an abbreviated version of the report and does not contain the full content. A link to download the full report is available above.
If you have difficulty obtaining a copy of the report, please contact us using the "Contact" link below.
- Deloitte, “Making Maintenance Smarter”
- Tech-Clarity, “Medical Device Manufacturer’s Selection Guide for 2018”
[post_title] => IoT Medical Device Monitoring (Buyer's Guide) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => medical-device-iot [to_ping] => [pinged] => [post_modified] => 2022-11-14 22:27:56 [post_modified_gmt] => 2022-11-15 03:27:56 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7596 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [12] => WP_Post Object ( [ID] => 7469 [post_author] => 2572 [post_date] => 2019-03-21 14:45:28 [post_date_gmt] => 2019-03-21 18:45:28 [post_content] =>
Tech-Clarity's Preparing for EU MDR explains the impact of the EU MDR on your product data. While complying to the EU MDR may require an investment, taking the steps now to prepare your product data will not only support EU MDR compliance, but will position your company to support future regulatory requirements. In addition, these investment will also lead to greater efficiencies that will enable you to have more bandwidth to focus on innovation and quality. This report provides recommended steps to help you prepare and how software technology will make it easier.
Please enjoy the summary* below.
For the full eBook, please visit our sponsor PTC (free of charge, registration required).
The EU MDR: A Challenge and an Opportunity
 In May 2017, the EU published the new European Union Medical Device Regulation (EU MDR). The new regulation replaces the MDD (Medical Device Directive) and went into force on May 25, 2017. Medical devices have three years to comply while in vitro diagnostics have five years.
In May 2017, the EU published the new European Union Medical Device Regulation (EU MDR). The new regulation replaces the MDD (Medical Device Directive) and went into force on May 25, 2017. Medical devices have three years to comply while in vitro diagnostics have five years.
The reasons for the new regulation are admirable. As stated, "This Regulation aims to ensure the smooth functioning of the internal market…[it] sets high standards of quality and safety for medical devices in order to meet common safety concerns..." Essentially, the EU wants to ensure that medical devices are safe, high quality, meet stated expectations, and improve patient health. The EU also wants to take advantage of the technological and scientific progress made over the last 20 years.
Better Devices, but There’s Work to Do
 While taking advantage of technology advancements will lead to greater efficiencies for medical device companies, becoming compliant will require work. The EU MDR wiki states, "The new EU Medical Device Regulation (EU MDR) is not radically different from the current Medical Device Directive (MDD). That's not to underestimate the amount of work that will be required to switch from the current MDD to the new EU MDR.”1 The new regulation is largely an extension of the existing regulation, but it requires far more data. In many ways, the new regulations require processes that medical device companies should be following anyway, but have been a challenge to implement, especially without the right technology.
While taking advantage of technology advancements will lead to greater efficiencies for medical device companies, becoming compliant will require work. The EU MDR wiki states, "The new EU Medical Device Regulation (EU MDR) is not radically different from the current Medical Device Directive (MDD). That's not to underestimate the amount of work that will be required to switch from the current MDD to the new EU MDR.”1 The new regulation is largely an extension of the existing regulation, but it requires far more data. In many ways, the new regulations require processes that medical device companies should be following anyway, but have been a challenge to implement, especially without the right technology.
The Opportunity
 Considering that every medical device company that wants to do business in Europe will be undergoing efforts to comply, this transition period represents an excellent opportunity to restructure processes in ways that are more efficient, but will also lead to more innovative and higher quality devices. The transition may be challenging, but those who are most successful will likely enjoy a competitive advantage.
Considering that every medical device company that wants to do business in Europe will be undergoing efforts to comply, this transition period represents an excellent opportunity to restructure processes in ways that are more efficient, but will also lead to more innovative and higher quality devices. The transition may be challenging, but those who are most successful will likely enjoy a competitive advantage.
While the scope of the new regulation is quite broad, its implications are especially significant for product teams who will – either directly or behind the scenes – participate in the product submissions process.
This eBook shares advice to prepare product data for regulatory submissions through a programmatic and extensible approach. In this view, EU-MDR compliance serves as a catalyst for implementing sound product design, change and configuration control best practices across your product development lifecycle.
Recommendations
To establish a foundation to comply with the EU MDR, medical device companies should look to adopt a product-centric approach. Based on this research and our experience, we recommend that medical device companies do the following to support the EU MDR and a product-centric approach:
- Use the EU MDR as an opportunity to improve product related processes to improve efficiency and create more bandwidth to focus on innovation and device quality.
- To meet the increased data requirements, consider a product-centric approach. A product-centric approach uses product data, specifically CAD models, as the primary design artifact rather than a PDF. This approach establishes traceability and breaks down silos between the engineering data and documentation, making it easier to collect the data for EU MDR submissions.
- A PLM system will support this a product-centric approach can serve as a foundation for EU MDR, a PLM system supports the digital thread to tie product information, decisions, and history together in a structured, integrated way that captures product innovation and knowledge throughout the product lifecycle.
To adopt a product-centric approach, consider the following steps:
- Obtain Executive support: An Executive is required to support adoption across multiple departments and make the initiative a priority.
- Create a product data hub: Your data needs to be accessed from a central location with traceability across the lifecycle. You can accomplish this with either a single database or a data hub that connects to multiple data sources. A PLM system with a connection platform will enable the latter option.
- Structure and manage data: Structure your product data so that it is centralized and you can find it. Consider starting with your CAD data.
- Identify a cross-functional team: Take advance of the collective expertise of your teams to help identify bottlenecks and opportunities for improvements.
- Automate Product Lifecycle Processes: Map out your current processes and then how you would like them to work. Look for opportunities for technology to automate the workflow to minimize the amount of manual work.
*This summary is an abbreviated version of the eBook and does not contain the full content. A link to download the full eBook is available above.
If you have difficulty obtaining a copy of the research, please contact us using the “Contact” link below.

 How can manufacturers tell when they've outgrown their Product Data Management system? What should they look for to replace it? Join Tech-Clarity's Jim Brown and PTC's Mark Lobo as they help companies navigate this all-too-common scenario. The interactive presentation will share what's missing in most basic PDM / Engineering Document Management (EDM) systems and what to look for in a more advanced PDM solution that does more than manage CAD, what some might call cPDM. The presentation shares Jim and Mark's experience as well as findings from a recent Buyer's Guide that shares functional requirements, implementation needs, vendor considerations, and other special concerns such as preparing for the future.
Watch the PTC sponsored webcast replay now (registration required).
How can manufacturers tell when they've outgrown their Product Data Management system? What should they look for to replace it? Join Tech-Clarity's Jim Brown and PTC's Mark Lobo as they help companies navigate this all-too-common scenario. The interactive presentation will share what's missing in most basic PDM / Engineering Document Management (EDM) systems and what to look for in a more advanced PDM solution that does more than manage CAD, what some might call cPDM. The presentation shares Jim and Mark's experience as well as findings from a recent Buyer's Guide that shares functional requirements, implementation needs, vendor considerations, and other special concerns such as preparing for the future.
Watch the PTC sponsored webcast replay now (registration required).
 For more information, please see our related Buyer's Guide, below:
https://tech-clarity.com/outgrown-pdm/7356
[post_title] => Top Ten Signs You've Outgrown Your PDM System (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => outgrown-pdm-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:52
[post_modified_gmt] => 2022-11-15 03:26:52
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7453
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[14] => WP_Post Object
(
[ID] => 7445
[post_author] => 2
[post_date] => 2019-02-19 11:13:39
[post_date_gmt] => 2019-02-19 16:13:39
[post_content] =>
For more information, please see our related Buyer's Guide, below:
https://tech-clarity.com/outgrown-pdm/7356
[post_title] => Top Ten Signs You've Outgrown Your PDM System (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => outgrown-pdm-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:52
[post_modified_gmt] => 2022-11-15 03:26:52
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7453
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[14] => WP_Post Object
(
[ID] => 7445
[post_author] => 2
[post_date] => 2019-02-19 11:13:39
[post_date_gmt] => 2019-02-19 16:13:39
[post_content] =>  What should Life Sciences Companies look for in a solution to transform service using IoT remote monitoring? Tech-Clarity's Jim Brown and PTC's Anthony Moffa will share recent research and industry experience on this Medical Design & Outsourcing webcast, How to Select a Remote Monitoring Solution to Transform Your Service Model. The webcast will cover how to improve service maturity to better service medical equipment, leading to both higher profitability and better patient outcomes. We'll also share how this initiative can set the foundation for even broader IoT and digitalization benefits.
What should Life Sciences Companies look for in a solution to transform service using IoT remote monitoring? Tech-Clarity's Jim Brown and PTC's Anthony Moffa will share recent research and industry experience on this Medical Design & Outsourcing webcast, How to Select a Remote Monitoring Solution to Transform Your Service Model. The webcast will cover how to improve service maturity to better service medical equipment, leading to both higher profitability and better patient outcomes. We'll also share how this initiative can set the foundation for even broader IoT and digitalization benefits.
 Register for the Medical Design & Sourcing webinar today (no charge, registration required).
Register for the Medical Design & Sourcing webinar today (no charge, registration required).
Event Synopsis
In the IoT era, service transformation is the new frontier of innovation. For life sciences and medical device innovators, remote monitoring is the superpower that unlocks service value through reduced service costs, improved customer satisfaction, and greater insight. When it comes to choosing the best remote monitoring software, medical device makers have many factors to consider. Fortunately, you don’t have to make the decision alone. In this upcoming February 28 discussion, Jim Brown and Anthony Moffa put remote monitoring under the microscope. Hear the crucial steps to choosing a remote monitoring solution and learn:- The three stages of connected services maturity.
- The critical role of software analytics – and what to look for.
- How to make the most of your IoT investment from device design all the way through to maintenance and service.
 Is your CAD tool ready for the 2020s? Does it have the features you need to keep your company competitive?
Tech-Clarity's How to Futureproof Your Product Design: 5 Technologies Your CAD Tool Must Support describes the key technologies that will impact design in the 2020s. Based on a survey of over 200 manufacturers, respondents identified the top technologies that will change how they design products. This report describes how these technologies will impact design and how a CAD tool should help you.
Please enjoy the summary* below.
For the full eBook, please visit our sponsor SOLIDWORKS (free of charge, registration required).
Is your CAD tool ready for the 2020s? Does it have the features you need to keep your company competitive?
Tech-Clarity's How to Futureproof Your Product Design: 5 Technologies Your CAD Tool Must Support describes the key technologies that will impact design in the 2020s. Based on a survey of over 200 manufacturers, respondents identified the top technologies that will change how they design products. This report describes how these technologies will impact design and how a CAD tool should help you.
Please enjoy the summary* below.
For the full eBook, please visit our sponsor SOLIDWORKS (free of charge, registration required).
Is Your CAD Tool Ready for the 2020s?

How Will You Stay Competitive in the 2020s?
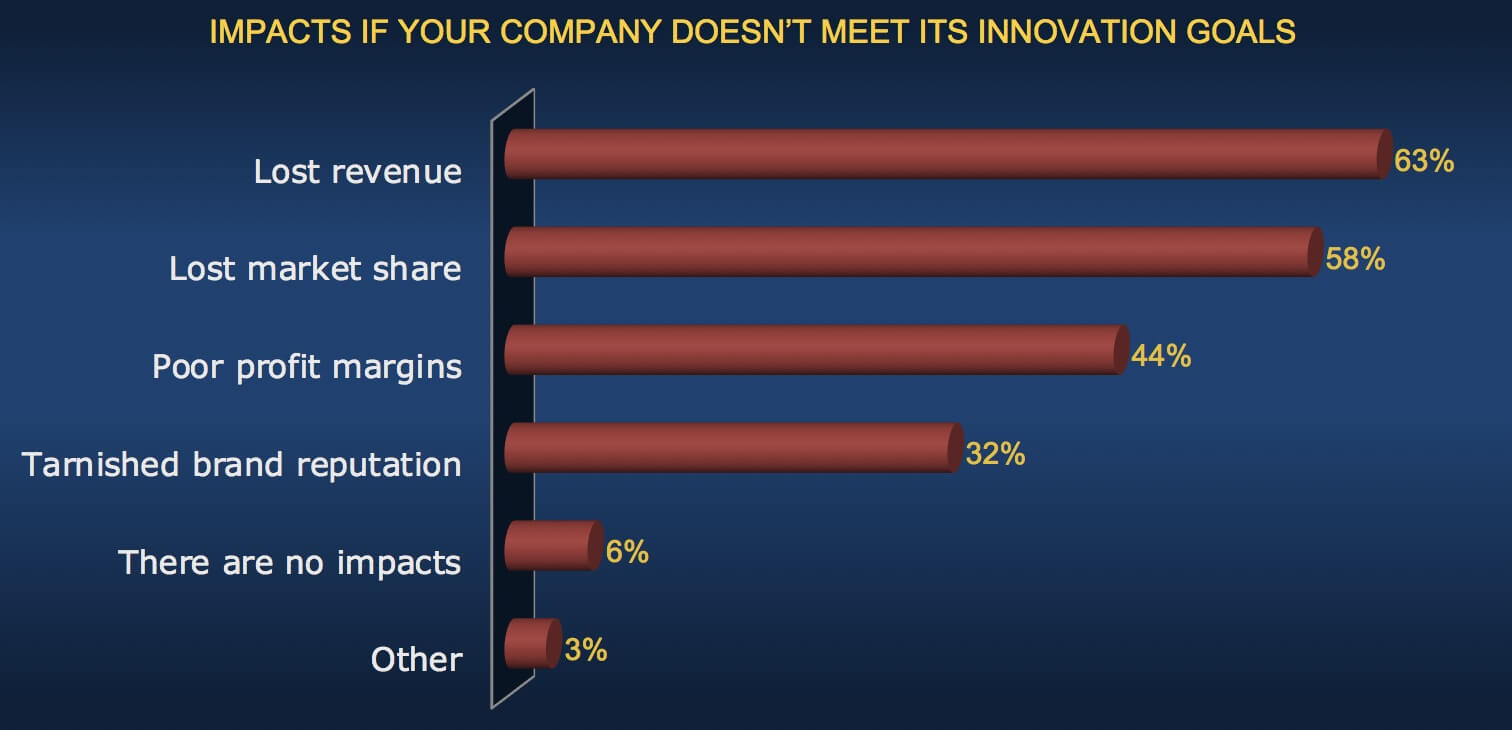 You Will Need the Right Software Tools
While the right strategy around cutting-edge technologies is critical to avoid these negative impacts, you must also empower your teams with proper processes and technology. Consequently, you also need capabilities to manage the resulting complexity. This research reveals what successful companies do to help them succeed and stay ahead of the competition while they prepare for the next decade.
*This summary is an abbreviated version of the eBook and does not contain the full content. A link to download the full eBook is available above.
If you have difficulty obtaining a copy of the research, please contact us using the “Contact” link below.
[post_title] => How to Futureproof Your Product Design (survey results)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-to-futureproof-your-product-design-survey-results
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:28:50
[post_modified_gmt] => 2022-11-15 03:28:50
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7422
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[16] => WP_Post Object
(
[ID] => 7395
[post_author] => 2
[post_date] => 2019-02-13 10:25:13
[post_date_gmt] => 2019-02-13 15:25:13
[post_content] =>
You Will Need the Right Software Tools
While the right strategy around cutting-edge technologies is critical to avoid these negative impacts, you must also empower your teams with proper processes and technology. Consequently, you also need capabilities to manage the resulting complexity. This research reveals what successful companies do to help them succeed and stay ahead of the competition while they prepare for the next decade.
*This summary is an abbreviated version of the eBook and does not contain the full content. A link to download the full eBook is available above.
If you have difficulty obtaining a copy of the research, please contact us using the “Contact” link below.
[post_title] => How to Futureproof Your Product Design (survey results)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-to-futureproof-your-product-design-survey-results
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:28:50
[post_modified_gmt] => 2022-11-15 03:28:50
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7422
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[16] => WP_Post Object
(
[ID] => 7395
[post_author] => 2
[post_date] => 2019-02-13 10:25:13
[post_date_gmt] => 2019-02-13 15:25:13
[post_content] =>  How are innovative automotive companies leveraging immersive, real-time 3D to improve innovation? How can they use highly realistic, interactive, virtual experiences to enhance design, engineering, manufacturing engineering, sales, marketing, training, and more? This eBook shares industry insights and experiences from Ford, Audi, Teague, Team One / Saatchi & Saatchi, and other manufacturers to shed light on the opportunity.
The Value of Immersive 3D Automotive Experiences eBook shares interviews with leading manufacturing, design, and marketing firms to offer insight into how auto companies can create compelling visual representations that excite buyers, inform engineers, optimize design decisions, and improve training. The research shares how companies are using tools from the gaming industry in combination with their CAD models to lower the barriers to creating these powerful, virtual capabilities. The eBook shares a variety of approaches manufacturers are adopting along with the business benefits they're achieving.
Please enjoy the summary below. For the full report, please visit our sponsor Unity (no charge, registration required).
How are innovative automotive companies leveraging immersive, real-time 3D to improve innovation? How can they use highly realistic, interactive, virtual experiences to enhance design, engineering, manufacturing engineering, sales, marketing, training, and more? This eBook shares industry insights and experiences from Ford, Audi, Teague, Team One / Saatchi & Saatchi, and other manufacturers to shed light on the opportunity.
The Value of Immersive 3D Automotive Experiences eBook shares interviews with leading manufacturing, design, and marketing firms to offer insight into how auto companies can create compelling visual representations that excite buyers, inform engineers, optimize design decisions, and improve training. The research shares how companies are using tools from the gaming industry in combination with their CAD models to lower the barriers to creating these powerful, virtual capabilities. The eBook shares a variety of approaches manufacturers are adopting along with the business benefits they're achieving.
Please enjoy the summary below. For the full report, please visit our sponsor Unity (no charge, registration required).

Table of Contents
- Automotive Innovators Gaining Value from Real-Time, Immersive 3D
- Add Excitement and Efficiency to Marketing and Sales
- Increase Insight and Iteration in Product Innovation and Design
- Add Realism and Immersion to R&D and Engineering
- Boost Productivity and Quality in Manufacturing Engineering
- Reduce Constraints and Add Interest to Training
- Unleash Innovation and Explore Additional Uses
- Roadblocks and Speed Bumps Removed
- The Automotive Industry Is Hitting the Accelerator
- Lead, Follow the Leaders, or Fall Further Behind
- About the Author
- Experience Automotive Emotion in Action
A car represents a unique blend of advanced technology and consumer desires, and automotive marketing campaigns are tailored to evoke both rational and emotional responses from customers. Auto companies and advertising agencies have become highly adept at showing lifelike driving experiences. Today’s customers have the opportunity to experience a car in a spectacular setting, even before a single unit has been produced.
Innovators Taking 3D Beyond the Basics
The innovators are doing even more, gaining value by sharing interactive, real-time 3D experiences across the enterprise and the supply chain. They can create buyer excitement with fully immersive configurators that customers can interactively explore in 3D. They can collaborate on interior designs in global, virtual reality design reviews to innovate and catch errors early. They can analyze the impact of manufacturing engineering decisions on perceived quality by viewing fit and finish in real-time under a variety of lighting conditions. They can even interactively experience airflow simulation results by stepping inside a virtual smoke stream. Or, they can train operators to assemble engines in augmented reality before the line is built and without expensive mockups.

Opportunities Span the Automotive Enterprise
The opportunities are extensive. This eBook examines the benefits along multiple dimensions, including the following:
- Marketing and Sales
- Product Innovation and Design
- R&D and Engineering
- Manufacturing Engineering
- Training
The eBook shares valuable insights from interviews with industry leaders Ford and Teague, and highlights from a recent industry conference with speakers from Audi, EDAG, and Team One. It concludes with some valuable insights to help auto companies better understand the opportunities and enabling technologies available to help them take advantage of real-time, interactive, immersive 3D.
Real-time 3D Is Coming, Ready or Not
The value of using real-time, immersive 3D in the automotive industry is compelling. The leaders will continue to innovate and expand on their lead. More importantly, lowered barriers to entry mean more companies will be able to take advantage of real-time 3D benefits, including startups that are embracing digitalization in order to displace industry veterans. Immersive, real-time 3D is quickly becoming a critical part of the modern technical toolkit for the automotive industry. We believe the innovators will continue to push forward, while those that don’t adopt immersive 3D technologies will fall further behind.
Getting Started
It’s time for companies to find an opportunity or a problem and address it. Companies should start small with a proof of concept (POC) as a first step. The POC, however, should provide value and not just serve as a learning exercise. Create value, learn, and build on that knowledge with next steps. Companies can choose a project similar to the ones described here, leveraging the experience of the pioneers, or unlock the creativity of their own organization by, for example, holding a “hackathon.”
The Time to Act Is Now
If your company has been waiting to get involved with real-time 3D, now is the time. The costs have dropped tremendously. The time required to build valuable solutions in 3D is much lower. Specialized development resources are now ready to go. Computing power is available onsite or via the cloud. Real-time, immersive 3D use cases have never been more in reach. Companies that adopt real-time 3D can still gain an advantage, while automotive companies can reap significant benefits in multiple dimensions of their business:
- Marketing and Sales
- Product Innovation and Design
- R&D and Engineering
- Manufacturing Engineering
- Training
- Autonomous Vehicle Training
- VR Analytics
During this on-demand webinar, Tech-Clarity's Michelle Boucher and Anshuman Prakash from Siemens share perspectives on better requirements management. They also provide advice to make the process easier. During the webinar, you will learn:
- How to address common challenges that lead to missed requirements
- How to easily assess the impact of proposed design changes
- What strategies can keep budgets in check when requirements change
- How to manage requirements across mechanical and electrical domains
 [post_title] => Better Requirements Management to Save Your Bottom Line (Webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => better-requirements-management
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:47
[post_modified_gmt] => 2022-11-15 03:26:47
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7392
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[18] => WP_Post Object
(
[ID] => 7382
[post_author] => 2572
[post_date] => 2019-01-21 11:04:05
[post_date_gmt] => 2019-01-21 16:04:05
[post_content] => Did you know that 9.9% of every dollar spent on projects is wasted due to poor project performance?
[post_title] => Better Requirements Management to Save Your Bottom Line (Webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => better-requirements-management
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:47
[post_modified_gmt] => 2022-11-15 03:26:47
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=7392
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[18] => WP_Post Object
(
[ID] => 7382
[post_author] => 2572
[post_date] => 2019-01-21 11:04:05
[post_date_gmt] => 2019-01-21 16:04:05
[post_content] => Did you know that 9.9% of every dollar spent on projects is wasted due to poor project performance?
 This startling statistic comes from research done by the Project Management Institute (PMI), published in their 2018 Pulse of the Profession® report. In a guest post on the Siemens Solid Edge blog, Michelle Boucher outlines some key reasons why a requirements management solution can help improve the performance of product development projects so that you can minimize or even avoid some of the challenges that contribute to wasted budget.
This startling statistic comes from research done by the Project Management Institute (PMI), published in their 2018 Pulse of the Profession® report. In a guest post on the Siemens Solid Edge blog, Michelle Boucher outlines some key reasons why a requirements management solution can help improve the performance of product development projects so that you can minimize or even avoid some of the challenges that contribute to wasted budget.
Requirements Management Is Critical to Project Success
When PMI dug deeper into the reasons for project failures, they found that the top three issues include changing priorities, changing objectives, and inaccurate requirements gathering. While these problems create substantial challenges, they can become easier to manage with improvements to requirements management. Requirements management has a significant impact on project performance. Getting it right can mean the difference between a successful new product development project or a failure.New Ways of Working Are Needed
So how do these issues impact requirements management? The critical point is adapting to change, which companies must do to survive in today's competitive global economy. For a new product development project, getting the design right starts with the requirements. However, markets are evolving so rapidly, no wonder there are changing priorities and objectives. Disruptive technologies are bringing new sources of innovation and changing how we do business. Alternative sources of funding like Kickstarter have lowered the barriers to entry for start-ups, and the internet and cloud technology have expanded companies’ global reach so that even small companies can operate like larger ones. Responding to these trends impacts requirements, but if you cannot adapt quickly, you risk losing market share. With today's changing market dynamics, old ways of working may no longer be enough. You may need to find new ways of working to be competitive Continue to the Siemens Solid Edge blog for the full post. For more information on requirements management, access the on demand webinar titled, Better Requirements Management to Save Your Bottom Line. (registration required). [post_title] => Better Requirements Management: An Immediate Payback (Guest Post) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => better_requirements_management [to_ping] => [pinged] => [post_modified] => 2022-11-14 22:26:06 [post_modified_gmt] => 2022-11-15 03:26:06 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7382 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [19] => WP_Post Object ( [ID] => 7369 [post_author] => 2 [post_date] => 2019-01-03 08:23:54 [post_date_gmt] => 2019-01-03 13:23:54 [post_content] => What challenges do manufacturers encounter when working with contract manufacturers? What should they do to reduce the frustration and streamline the relationship?
Jim Brown introduces five questions you may find yourself asking if you're working with a CMO, CDMO, or other supply chain partners and then offers recommendations based on our experience and research in this guest post on the upchain blog. Read the CM Conundrum post on the upchain site now (no charge, no registration required).
What challenges do manufacturers encounter when working with contract manufacturers? What should they do to reduce the frustration and streamline the relationship?
Jim Brown introduces five questions you may find yourself asking if you're working with a CMO, CDMO, or other supply chain partners and then offers recommendations based on our experience and research in this guest post on the upchain blog. Read the CM Conundrum post on the upchain site now (no charge, no registration required).
Introduction
Working with contract manufacturers can be one of those “you can’t live with them, you can’t live without them” scenarios. Leveraging contract manufacturing organizations (CMO) is a reality for many companies and can be a great way to jumpstart a product when you don’t have the time or capital to bring up your own plants. What’s more, manufacturers with complex, digital products like telecommunications or other electronic devices may not have another viable option. They simply can’t get from prototype to scale on their own given the required time, money, and manufacturing knowhow. But working with third parties can be frustrating, particularly if you’re used to making all of the calls and you’re new to the process. It doesn’t seem like it should be so hard, but there’s a lot that can go wrong – and it usually does. You may find yourself asking some frustrating questions, but there are ways to manage the inherent challenges and be successful. Let’s explore five questions you may find yourself asking during your CMO partnership, and what you can do about them. Continue to the upchain site for the full post. [post_title] => 5 Frustrations of Working with Contract Manufacturers (guest post) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => contract-mfg-challenges [to_ping] => [pinged] => [post_modified] => 2022-11-14 22:25:36 [post_modified_gmt] => 2022-11-15 03:25:36 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=7369 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) ) [post_count] => 20 [current_post] => -1 [before_loop] => 1 [in_the_loop] => [post] => WP_Post Object ( [ID] => 7936 [post_author] => 2 [post_date] => 2019-06-04 09:34:12 [post_date_gmt] => 2019-06-04 13:34:12 [post_content] => How can manufacturers use the digital twin and industrial IoT to dramatically improve manufacturing and product performance? This eBook shares the value of the digital twin and IIoT for products and in the plant. Then, it shares some practical examples and advice to get started down the path to improved performance, availability, and product quality. Please enjoy the eBook content below or download a PDF of the eBook here (no registration required) courtesy of our sponsor, Siemens Industry Software (formerly known as Siemens PLM Software). You can also learn more from Siemens about the Industrial Internet of Things on their website.Disruption Demands Digitalization and the Digital Twin
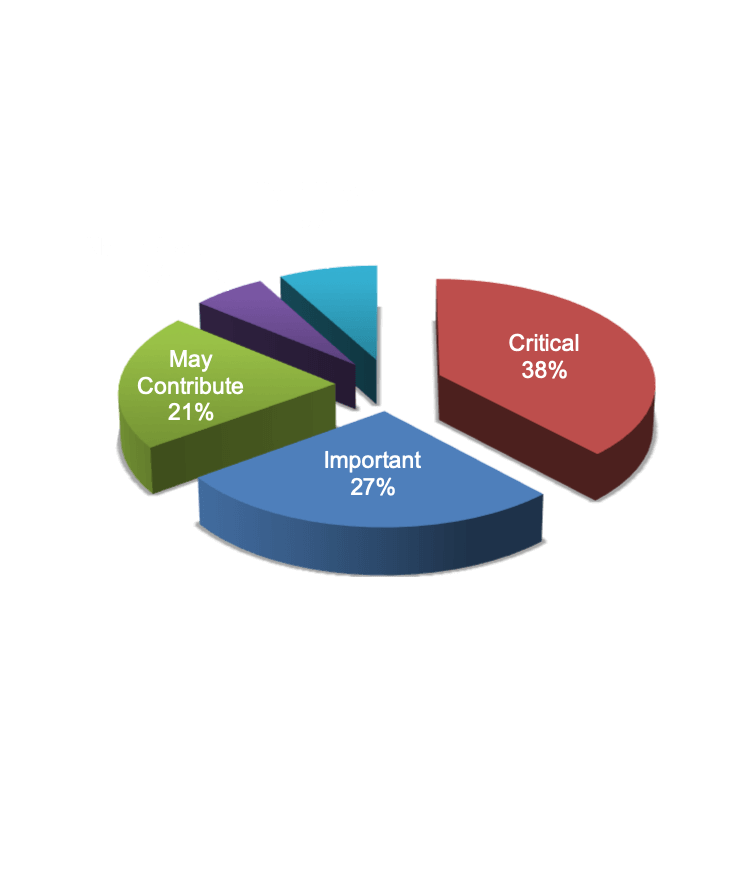 Manufacturing is Undergoing a Revolution
The manufacturing industries are getting more challenging. Manufacturers must evolve as new technologies remove barriers to entry and enable new, digital players to challenge market share. Operational efficiency is no longer enough to compete in today’s era of digitalization and Industry 4.0.
To remain competitive, companies have to maintain high productivity while offering unprecedented levels of flexibility and responsiveness. We believe this is a fundamental disruption that will change the status quo. To survive, manufacturers need to digitalize operations in order to improve speed, agility, quality, costs, customer satisfaction, and the ability to tailor to customer and market needs.
Raising the Bar with Digitalization: The Digital Twin
One of the most compelling digitalization opportunities is adopting the digital twin. This approach combines a number of digital technologies to significantly improve quality and productivity. It starts with comprehensive, virtual models of physical assets – products and production lines – to help optimize designs. But the value is much greater because the physical and virtual twins are connected and kept in sync with real data from the Internet of Things (IoT) and Industrial IoT (IIoT). Further, companies can use analytics to analyze digital twin data to develop deep insights and intelligence that allow for real-time intervention and long-term, continuous improvement.
The Digital Twin’s Impact on the Plant
The digital twin holds significant productivity and quality opportunities for the plant. It can be used to understand when the plant isn’t operating as intended. It can identify or predict equipment issues that can result in unplanned downtime or correct process deviations before they result in quality slippage, scrap, and rework. In addition, it can significantly further benefits already achieved by six sigma programs and support greater continuous improvement. Let’s learn how manufacturers can leverage the digital twin to improve plant performance and product quality.
Manufacturing is Undergoing a Revolution
The manufacturing industries are getting more challenging. Manufacturers must evolve as new technologies remove barriers to entry and enable new, digital players to challenge market share. Operational efficiency is no longer enough to compete in today’s era of digitalization and Industry 4.0.
To remain competitive, companies have to maintain high productivity while offering unprecedented levels of flexibility and responsiveness. We believe this is a fundamental disruption that will change the status quo. To survive, manufacturers need to digitalize operations in order to improve speed, agility, quality, costs, customer satisfaction, and the ability to tailor to customer and market needs.
Raising the Bar with Digitalization: The Digital Twin
One of the most compelling digitalization opportunities is adopting the digital twin. This approach combines a number of digital technologies to significantly improve quality and productivity. It starts with comprehensive, virtual models of physical assets – products and production lines – to help optimize designs. But the value is much greater because the physical and virtual twins are connected and kept in sync with real data from the Internet of Things (IoT) and Industrial IoT (IIoT). Further, companies can use analytics to analyze digital twin data to develop deep insights and intelligence that allow for real-time intervention and long-term, continuous improvement.
The Digital Twin’s Impact on the Plant
The digital twin holds significant productivity and quality opportunities for the plant. It can be used to understand when the plant isn’t operating as intended. It can identify or predict equipment issues that can result in unplanned downtime or correct process deviations before they result in quality slippage, scrap, and rework. In addition, it can significantly further benefits already achieved by six sigma programs and support greater continuous improvement. Let’s learn how manufacturers can leverage the digital twin to improve plant performance and product quality.
How the Digital Twin Drives Value
 Develop Comprehensive Virtual Models (the Virtual Twin)
First, let’s step back to discuss what a digital twin is and, more importantly, how it creates business value. Digital twins consist of a virtual model and a physical item that are identical. At the core, the virtual component of the twin is a model that is rich enough to simulate and predict the physical item’s behavior. These representations can be used to optimize and validate designs early in the product or asset lifecycle and are subsequently kept in sync with the physical item throughout its life.
The digital twin concept can be applied to a number of different physical items:
Develop Comprehensive Virtual Models (the Virtual Twin)
First, let’s step back to discuss what a digital twin is and, more importantly, how it creates business value. Digital twins consist of a virtual model and a physical item that are identical. At the core, the virtual component of the twin is a model that is rich enough to simulate and predict the physical item’s behavior. These representations can be used to optimize and validate designs early in the product or asset lifecycle and are subsequently kept in sync with the physical item throughout its life.
The digital twin concept can be applied to a number of different physical items:
- Products (equipment, devices, etc.)
- Production (machines, lines, plants)
- Infrastructure (cities, etc.)
- Something wrong with the physical asset that can be corrected
- Something wrong with the virtual model or simulation that leads to new understanding and provides knowledge for future modeling and simulation efforts
Applying Virtual Twins in the Manufacturing Industry
Target the Right Virtual Twins for Manufacturing There are many ways to gain value by applying the digital twin approach in the manufacturing industries. In the plant, the typical ways to create value are from creating twins for products and for production assets. Let’s discuss how to create and drive value from each of these. Develop Virtual Twins of Products
The first way many people think of the digital twin is as a digital model of a product. This typically starts with a 3D CAD model, but also captures much of the information captured in model-based design approaches. For today’s smart products, the model typically incorporates electronic, software, and systems designs.
The virtual product twin can be used to simulate and communicate planned product behavior and predict product characteristics like throughput, torque, energy consumption, or other performance characteristics. To do this, the virtual twin must accurately represent a specific configuration or unit of the product and be kept up to date with changes. The product model should also include production requirements to support a design anywhere / produce anywhere strategy.
Create Virtual Twins of the Plant
One manufacturer’s product may be the equipment in another’s plant. The virtual model concept extends to equipment, work stations, lines, and entire plants. These twins should completely model production at the appropriate level of granularity for their intended purpose.
The twin can also include the virtual twin of products being produced and the operators running the plant.
These models can be used to simulate and optimize production plans, plant layouts, materials flows, and equipment. Manufacturers can use the models to virtually operate the plant all the way down to control code in order to detect collisions, bottlenecks, and analyze throughput. This allows them to optimize production behavior virtually to get designs right before making physical investments.
Finally, virtual twins can be used to develop code for CNC equipment that can be run and validated on virtual PLCs using an approach known as “software in the loop.” This code can then be used for virtual commissioning to speed ramp up and changeovers.
Develop Virtual Twins of Products
The first way many people think of the digital twin is as a digital model of a product. This typically starts with a 3D CAD model, but also captures much of the information captured in model-based design approaches. For today’s smart products, the model typically incorporates electronic, software, and systems designs.
The virtual product twin can be used to simulate and communicate planned product behavior and predict product characteristics like throughput, torque, energy consumption, or other performance characteristics. To do this, the virtual twin must accurately represent a specific configuration or unit of the product and be kept up to date with changes. The product model should also include production requirements to support a design anywhere / produce anywhere strategy.
Create Virtual Twins of the Plant
One manufacturer’s product may be the equipment in another’s plant. The virtual model concept extends to equipment, work stations, lines, and entire plants. These twins should completely model production at the appropriate level of granularity for their intended purpose.
The twin can also include the virtual twin of products being produced and the operators running the plant.
These models can be used to simulate and optimize production plans, plant layouts, materials flows, and equipment. Manufacturers can use the models to virtually operate the plant all the way down to control code in order to detect collisions, bottlenecks, and analyze throughput. This allows them to optimize production behavior virtually to get designs right before making physical investments.
Finally, virtual twins can be used to develop code for CNC equipment that can be run and validated on virtual PLCs using an approach known as “software in the loop.” This code can then be used for virtual commissioning to speed ramp up and changeovers.
Creating the Complete Digital Twin in Manufacturing
 Gain Greater Value from Connected Digital Twins
The value of digital twins specific to manufacturing is greatly enhanced when the virtual and physical twins are connected via the IIoT. Manufacturer operations can produce a lot of data. For example, companies may already have MES and other control data, but they traditionally have trouble unlocking the value from it. The value of this data is made tangible when it’s put into the context of the digital model. Connecting the virtual and physical twins allows companies to compare actual to expected results and behavior of plants, product lines, work cells, machines, and even the products produced. The digital twin helps them gain intelligence from the data instead of simply storing it away.
Connect and Analyze Plant and Product Virtual Twins
Manufacturers can learn a lot from discrepancies between expected performance predicted using the virtual twin and measured performance of the physical twin. The twin defines what “good” looks like in production and what “right” looks like with the products. Variances from simulated values may identify issues in the plant that can be diagnosed and remedied to bring performance back into plan.
A more complete digital approach leverages analytics and the cloud. Analytics creates deeper insights to gain product and process intelligence. Let’s take a look at how the intelligence derived from the digital twin drives tangible business value for manufacturers.
Gain Greater Value from Connected Digital Twins
The value of digital twins specific to manufacturing is greatly enhanced when the virtual and physical twins are connected via the IIoT. Manufacturer operations can produce a lot of data. For example, companies may already have MES and other control data, but they traditionally have trouble unlocking the value from it. The value of this data is made tangible when it’s put into the context of the digital model. Connecting the virtual and physical twins allows companies to compare actual to expected results and behavior of plants, product lines, work cells, machines, and even the products produced. The digital twin helps them gain intelligence from the data instead of simply storing it away.
Connect and Analyze Plant and Product Virtual Twins
Manufacturers can learn a lot from discrepancies between expected performance predicted using the virtual twin and measured performance of the physical twin. The twin defines what “good” looks like in production and what “right” looks like with the products. Variances from simulated values may identify issues in the plant that can be diagnosed and remedied to bring performance back into plan.
A more complete digital approach leverages analytics and the cloud. Analytics creates deeper insights to gain product and process intelligence. Let’s take a look at how the intelligence derived from the digital twin drives tangible business value for manufacturers.
Improve Equipment Performance and Availability
Monitor and Optimize Equipment Performance The first way we’ll examine the business value of the digital twin in the plant is by improving equipment performance. Today’s manufacturers need to get the most out of available production assets. Manufacturers can use the digital twin to monitor equipment and production via the IIoT. They can aggregate, filter, and analyze sensor and control data in the context of the virtual twin to find issues. The resultant information can be shared in dashboards and put into action with alerts so issues like reduced production rates can be addressed before they arise or escalate. Predict and Improve Equipment Availability One of the most promising ways manufacturers improve productivity is through predictive analytics. Instead of maintaining production equipment on estimated service intervals, manufacturers can monitor equipment to identify when it begins to show signs of wear or failure, such as higher temperatures or vibration. But analytics can also find hidden patterns, anomalies, or trends in operational data that may indicate a pending equipment failure. Transitioning to predictive service allows manufacturers to reduce downtime and avoid the cost of unnecessarily taking equipment offline for maintenance when it’s not necessary. Of course, not every issue can be predicted, and in those cases IoT and the digital twin can provide the intelligence needed to improve issue identification, reduce repair time, and analyze root causes. The resulting enhanced productivity from better equipment performance and availability directly drives improved profitability.
Improve Product Quality
 Monitor and Maintain Product Quality
The next way that digital twins offer tangible business value to plants is by improving quality. Manufacturers can use the IIoT to monitor production and compare it to the digital twin to find goods that are trending out of spec. For example, they could compare product tolerances with IIoT feeds from metrology devices to find anomalies. Then, they can use trend charts, histograms, or dashboards with alerts to identify potential quality slippage.
Control and Assure Process Quality
In addition to measuring production output, manufacturers can monitor the production process to build quality in. They can collect KPIs like cycle times or track control parameters to identify when processes aren’t fully in control so they can intervene.
Manufacturers can also use analytics to understand the relationship between variability in production specifications and output to help identify factors that lead to quality slippage. They can use artificial intelligence (AI) to gain insights from a combination of enterprise system data, environmental conditions, operator information, equipment setup, and machine settings. This can help them close the loop between engineering and actual resulting product performance characteristics, for example finding relationships between equipment settings or environmental factors and defects. They can also leverage AI to identify early indicators that production isn’t behaving as expected in order to identify and correct errors in real time.
Monitor and Maintain Product Quality
The next way that digital twins offer tangible business value to plants is by improving quality. Manufacturers can use the IIoT to monitor production and compare it to the digital twin to find goods that are trending out of spec. For example, they could compare product tolerances with IIoT feeds from metrology devices to find anomalies. Then, they can use trend charts, histograms, or dashboards with alerts to identify potential quality slippage.
Control and Assure Process Quality
In addition to measuring production output, manufacturers can monitor the production process to build quality in. They can collect KPIs like cycle times or track control parameters to identify when processes aren’t fully in control so they can intervene.
Manufacturers can also use analytics to understand the relationship between variability in production specifications and output to help identify factors that lead to quality slippage. They can use artificial intelligence (AI) to gain insights from a combination of enterprise system data, environmental conditions, operator information, equipment setup, and machine settings. This can help them close the loop between engineering and actual resulting product performance characteristics, for example finding relationships between equipment settings or environmental factors and defects. They can also leverage AI to identify early indicators that production isn’t behaving as expected in order to identify and correct errors in real time.
Next Steps
The Digital Industrial Revolution Requires Action Today’s manufacturers need to deliver high operational efficiency in order to compete. It’s time for them to digitalize to gain important benefits – agility, productivity, quality, cost, customer satisfaction, and responsiveness – and remain competitive into the future. Manufacturers can apply the digital twin concept to products, plants, or both to drive new levels of quality and productivity. The digital twin and the IIoT are key digitalization enablers with tangible, proven value. Get Started
To get started, manufacturers can choose between a number of valuable initiatives to leverage the digital twin, IIoT, and analytics. For example, they can use the digital twin, IIoT, and analytics to:
Get Started
To get started, manufacturers can choose between a number of valuable initiatives to leverage the digital twin, IIoT, and analytics. For example, they can use the digital twin, IIoT, and analytics to:
- Gain equipment intelligence and improve uptime
- Shift from preventative to predictive maintenance
- Monitor production to rapidly identify and correct quality or productivity issues
- Leverage actual results from the physical twin to improve simulations by feeding the digital twin with observed production scenarios and parameters
- Explore new business models by applying digital twin capabilities to their own products
All Results for "All"
Retail Predictive Analytics Solution (Buyer’s Checklist)
If you are a retail company, what should you look for in an analytics solution? In the retail industry, there is little room for error. Unfortunately, just a few bad seasons can put the entire business at risk. Consequently, you need to make fast decisions, identify opportunities, adapt to supply chain volatility, and more. How…
Empowering Design Engineers
How can you empower design engineers to make more informed decisions that can help set your products apart from the competition? Tech-Clarity’s Empowering Design Engineer infographic helps answer this question based on a survey of 195 companies. The infographic reveals top qualities that will make products more competitive over the next five years. These product…
The Five Basics of Effective BOM Processes (eBook)
BOM Processes Rely on Inadequate Solutions Ineffective BOM Processes Cause Disruption BOM management is critical to connecting design, purchasing, and production across a manufacturing business. Ineffective BOM processes, though, lead to low productivity and costly errors. With that in mind, why do so many manufacturers rely on substandard BOM management approaches like spreadsheets and email?…
How to Avoid Non-Value Added Work for Engineers (guest post)
Imagine what you could do if you could get almost a third of your time back? In a guest post on the Sodius Willert blog, Michelle Boucher shares research on how much engineering time is wasted on non-value added work and explains what you can do. Focus on the Work You Enjoy As an engineer,…
Retail Analytics Buyer’s Guide (eBook)
How do you navigate complex, omnichannel environments and manage multiple sources of data to make the best decisions? Can predictive analytics help? Tech-Clarity’s Retail Analytics Buyer’s Guide explains how predictive analytics provide better visibility across your retail business so that you can make better decisions to become more competitive. As the retail industry faces significant…
The Renaissance of CAD
As our changing world impacts product design, how should CAD evolve? How can CAD support your efforts to adopt some of the latest technologies? During the PTC on-demand virtual session, “The Renaissance of CAD: What’s New, What’s Now & What You Can Do With It” you will hear about some of the latest advancements in…
IoT, Industrial IoT, and Digital Twins in Food & Beverage Production (animation)
How can food and beverage companies use digitalization to dramatically increase flexibility, enhance consumer responsiveness, and improve productivity? This episode of Tech-Clarity TV, Revolutionizing Food and Beverage Production with the Industrial IoT and the Digital Twin, shares how food producers can leverage digital twins and the IoT to improve performance. This video series is sponsored…
Ten Practices to Successfully Implement Technology (eBook)
How can you get the quickest return on your investments in technology? As we look ahead to the coming decade, technology will become increasingly critical for your company to stay competitive. New technology can include CAD, CAE, PLM, 3D printing, IoT, and more. However, for that technology to work for your business, your implementation must…
Why Not Adopt Cloud for Product Innovation and Engineering? (guest post)
How have manufacturers’ opinions on using the cloud to support product innovation, product development, and engineering changed? How does that impact cloud adoption? Read the guest post in full in the Digital Transformation section of the Siemens PLM Community blog. Jim Brown recently shared a guest post on the Siemens’ blog offering our experience on…
Revolutionizing Simulation For Design Engineers
How do you empower engineers to design the best products possible? Research from Tech-Clarity’s Revolutionizing Simulation For Design Engineers research report finds that design engineers lack confidence in design decisions 28% of the time. The research identifies the most common ways engineers deal with this uncertainty, its impact, and how to improve confidence. This research…
IoT Medical Device Monitoring (Buyer’s Guide)
What should Life Sciences companies look for in a solution to help them simultaneously improve profitability and patient outcomes using remote device monitoring via the IoT? Our new Improving Service Performance and Patient Outcomes with Remote Monitoring Buyer’s Guide helps Life Sciences companies understand the business and social value of monitoring equipment with the IoT….
Preparing for EU MDR (eBook)
Are you ready for the EU MDR? Tech-Clarity’s Preparing for EU MDR explains the impact of the EU MDR on your product data. While complying to the EU MDR may require an investment, taking the steps now to prepare your product data will not only support EU MDR compliance, but will position your company to…
Top Ten Signs You’ve Outgrown Your PDM System (webcast)
How can manufacturers tell when they’ve outgrown their Product Data Management system? What should they look for to replace it? Join Tech-Clarity’s Jim Brown and PTC‘s Mark Lobo as they help companies navigate this all-too-common scenario. The interactive presentation will share what’s missing in most basic PDM / Engineering Document Management (EDM) systems and what…
Remote Monitoring Solution Selection for Medical Device Service (webcast)
What should Life Sciences Companies look for in a solution to transform service using IoT remote monitoring? Tech-Clarity’s Jim Brown and PTC’s Anthony Moffa will share recent research and industry experience on this Medical Design & Outsourcing webcast, How to Select a Remote Monitoring Solution to Transform Your Service Model. The webcast will cover how…
How to Futureproof Your Product Design (survey results)
Is your CAD tool ready for the 2020s? Does it have the features you need to keep your company competitive? Tech-Clarity’s How to Futureproof Your Product Design: 5 Technologies Your CAD Tool Must Support describes the key technologies that will impact design in the 2020s. Based on a survey of over 200 manufacturers, respondents identified…
The Value of Immersive 3D Automotive Experiences (eBook)
How are innovative automotive companies leveraging immersive, real-time 3D to improve innovation? How can they use highly realistic, interactive, virtual experiences to enhance design, engineering, manufacturing engineering, sales, marketing, training, and more? This eBook shares industry insights and experiences from Ford, Audi, Teague, Team One / Saatchi & Saatchi, and other manufacturers to shed light…
Better Requirements Management to Save Your Bottom Line (Webcast)
Does your company excel at meeting and managing product requirements? If not, this webinar will help you develop better requirements management practices. During this on-demand webinar, Tech-Clarity’s Michelle Boucher and Anshuman Prakash from Siemens share perspectives on better requirements management. They also provide advice to make the process easier. During the webinar, you will learn:…
Better Requirements Management: An Immediate Payback (Guest Post)
Did you know that 9.9% of every dollar spent on projects is wasted due to poor project performance? This startling statistic comes from research done by the Project Management Institute (PMI), published in their 2018 Pulse of the Profession® report. In a guest post on the Siemens Solid Edge blog, Michelle Boucher outlines some key reasons why…
5 Frustrations of Working with Contract Manufacturers (guest post)
What challenges do manufacturers encounter when working with contract manufacturers? What should they do to reduce the frustration and streamline the relationship? Jim Brown introduces five questions you may find yourself asking if you’re working with a CMO, CDMO, or other supply chain partners and then offers recommendations based on our experience and research in…