Does having multiple systems to manage product information create challenges for your company? Do you find some systems are so heavily customized, it’s hard to take advantage of the latest software enhancements? How can a PLM medical device solution help? If you have thought about any of these questions, you will find this webinar especially…
- The PLM journey taken by Boston Scientific
- What drove Boston Scientific to consolidate PLM systems
- How Boston Scientific executed their strategy for a unified PLM system
- Selection criteria you should look for in a PLM solution, especially for medical device companies
- Advice to improve product development efficiencies and support regulatory compliance
 [post_title] => How Boston Scientific Selected their PLM Medical Device Software Solution (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => boston-scientific-plm-medical-device
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6740
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[1] => WP_Post Object
(
[ID] => 6732
[post_author] => 2572
[post_date] => 2018-03-05 11:56:22
[post_date_gmt] => 2018-03-05 16:56:22
[post_content] =>
[post_title] => How Boston Scientific Selected their PLM Medical Device Software Solution (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => boston-scientific-plm-medical-device
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6740
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[1] => WP_Post Object
(
[ID] => 6732
[post_author] => 2572
[post_date] => 2018-03-05 11:56:22
[post_date_gmt] => 2018-03-05 16:56:22
[post_content] => Listen to, PTC's Dave Duncan and Tech-Clarity's Michelle Boucher discuss how to improve the accuracy of service information for field personnel. They share stories about Embraer, Airbus Helicopters, and Kirloskar Oil Engines and explain how they are transforming their service organizations. During this webcast, you will also learn:
- How poor service information affects technician productivity, customer satisfaction, and after-sales operations
- Best practices for producing accurate service documentation based on Tech-Clarity research
- What you can do to keep service manuals up-to-date so that field technicians can trust service documentation
- Tips to support the cultural changes required to support the transformation for a more service oriented company
 [post_title] => Service Information: The Pivotal Factor in Your Aftermarket Business (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => service-information-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6732
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[2] => WP_Post Object
(
[ID] => 6721
[post_author] => 2
[post_date] => 2018-02-28 08:48:20
[post_date_gmt] => 2018-02-28 13:48:20
[post_content] => How can digitalization help medical device companies improve innovation and drive profitable growth despite increased complexity and regulatory scrutiny? This animated video offers a look at what a digital medical device company looks like and offers three initiatives companies can adopt to start their digital transformation.
Digitalization offers significant benefits to medical device manufacturers because the status quo is no longer good enough. It relies too much on paper and electronic documents, inevitability leading to missed deadlines, mistakes, and inefficiency. In today's environment, key contributors work independently on their own information, leading to integration errors, compliance issues, and market delays. Now, companies can transform their businesses so that each step in the development process leverages digital information from the prior step, takes advantage of comprehensive 3D product models, and creates a complete digital thread. Digitalization can then extend into manufacturing and enable device manufacturers to quickly commission production and track manufacturing.
Medical Device companies have the opportunity to leverage digitalization and the digital enterprise to develop innovative products and bring them to market quickly. By digitalizing designs and workflows, digital medical device companies can:
[post_title] => Service Information: The Pivotal Factor in Your Aftermarket Business (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => service-information-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6732
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[2] => WP_Post Object
(
[ID] => 6721
[post_author] => 2
[post_date] => 2018-02-28 08:48:20
[post_date_gmt] => 2018-02-28 13:48:20
[post_content] => How can digitalization help medical device companies improve innovation and drive profitable growth despite increased complexity and regulatory scrutiny? This animated video offers a look at what a digital medical device company looks like and offers three initiatives companies can adopt to start their digital transformation.
Digitalization offers significant benefits to medical device manufacturers because the status quo is no longer good enough. It relies too much on paper and electronic documents, inevitability leading to missed deadlines, mistakes, and inefficiency. In today's environment, key contributors work independently on their own information, leading to integration errors, compliance issues, and market delays. Now, companies can transform their businesses so that each step in the development process leverages digital information from the prior step, takes advantage of comprehensive 3D product models, and creates a complete digital thread. Digitalization can then extend into manufacturing and enable device manufacturers to quickly commission production and track manufacturing.
Medical Device companies have the opportunity to leverage digitalization and the digital enterprise to develop innovative products and bring them to market quickly. By digitalizing designs and workflows, digital medical device companies can:
- Connect data and processes across disciplines and the lifecycle
- Leverage digital data from one step to the next
- Streamline engineering
- Reduce errors
- Improve traceability
- Digital design for medical device companies (overview)
- Digital design
- Digital design transfer
- Paperless manufacturing
 What should manufacturers look for when they buy cloud software for Product Lifecycle Management? Our PLM Cloud Buyer's Guide shares the important factors companies need to know when they evaluate cloud solutions. The guide explains the reasons companies are pursuing cloud options including standard benefits like reduced implementation cost and faster time to benefit, but also highlights specific advantages to product developers including the ability to better support global environments and to design-anywhere, build-anywhere. The eBook also shares the benefits for IT departments such as the ability to provide cloud-level performance and offload operational overhead to focus on more value-added activities. But despite the benefits of the cloud, the guide explains the need to have a fully capable PLM system as the highest priority before prioritizing the deployment option, what we call a solution-first approach.
As with our other Buyer's Guides, the guide goes beyond software requirements to share deployment options, pricing considerations, implementation factors, vendor considerations, and other special requirements such as industry needs. The guide also points out the need to select a solution and a partner that will serve as the foundation for continued growth as PLM continues to expand as the digital innovation backbone and serves the future needs of the digital enterprise with IoT, AR, the digital twin, and more.
Please enjoy the summary below, or click the report to download a PDF overview (free of charge, no registration required).
For the full Buyer's Guide, please visit our sponsor PTC.
What should manufacturers look for when they buy cloud software for Product Lifecycle Management? Our PLM Cloud Buyer's Guide shares the important factors companies need to know when they evaluate cloud solutions. The guide explains the reasons companies are pursuing cloud options including standard benefits like reduced implementation cost and faster time to benefit, but also highlights specific advantages to product developers including the ability to better support global environments and to design-anywhere, build-anywhere. The eBook also shares the benefits for IT departments such as the ability to provide cloud-level performance and offload operational overhead to focus on more value-added activities. But despite the benefits of the cloud, the guide explains the need to have a fully capable PLM system as the highest priority before prioritizing the deployment option, what we call a solution-first approach.
As with our other Buyer's Guides, the guide goes beyond software requirements to share deployment options, pricing considerations, implementation factors, vendor considerations, and other special requirements such as industry needs. The guide also points out the need to select a solution and a partner that will serve as the foundation for continued growth as PLM continues to expand as the digital innovation backbone and serves the future needs of the digital enterprise with IoT, AR, the digital twin, and more.
Please enjoy the summary below, or click the report to download a PDF overview (free of charge, no registration required).
For the full Buyer's Guide, please visit our sponsor PTC.
Cloud PLM Adoption and Buyer's Guide Introduction
Although manufacturers have started to adopt cloud solutions for many aspects of their business, Product Lifecycle Management (PLM) has lagged behind. The transition, however, is picking up pace. More companies are considering cloud PLM and many already leverage the cloud to get more value from PLM (and achieve that value faster).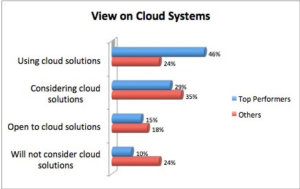 Given the increased interest and adoption, it’s time to put together some selection criteria to help companies with their decisions. We recommend that companies pick their solution first and then choose their deployment option. This is effectively a “solution first” approach as opposed to a “cloud first” approach. We find that companies are just not willing to shortchange functionality in this crucial area. They recognize it’s important to evaluate the functional capabilities of a PLM system to ensure they’ll gain the significant top- and bottom-line benefits that PLM delivers.
There are still important decisions to make after a solution is selected. Many systems can be deployed in a variety of ways ranging from cloud Software as a Service (SaaS) to traditional, on-premise implementations. The deployment choice impacts important factors including cost, security, resource requirements, performance, risk, and time to benefit. This guide is designed to help companies navigate the options and choose the best-suited PLM cloud option for their business.
*This summary is an abbreviated version of the report and does not contain the full content. A link to download the full report is available above.
[post_title] => Cloud PLM Buyer's Guide
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-plm-guide
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:27:54
[post_modified_gmt] => 2022-11-15 03:27:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6708
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[4] => WP_Post Object
(
[ID] => 6700
[post_author] => 2
[post_date] => 2018-02-20 11:20:02
[post_date_gmt] => 2018-02-20 16:20:02
[post_content] => Medical Device companies have to manage complexity and regulation without placing excess overhead on their designers. Devices complexity has led to increased complaints, CAPAs, observations, and recalls. The root cause is frequently insufficient and cumbersome design control.
Digitalization helps them design with agility while maintaining control. It improves speed, accuracy, and productivity by automating submissions, DMRs, and DHFs from design data. The digital approach transitions device manufacturers away from document-centric and paper-based processes, or the electronic equivalent of processes that originated on paper that have been automated but never digitalized.
Digital design leverages complete digital models and creates a complete digital thread of information through design and the product lifecycle. It allows a cohesive approach where each step in design builds on the previous, and results in a digital model that engineers can use to validate and verify the behavior of the physical product in digital form using simulation.
Digital design control helps medical device companies get devices approved more quickly, keeps quality high, reduces risk, controls cost, and helps companies get innovative product to market quickly. The results are faster time to market, increased quality, and improved compliance.
This video series is sponsored by Siemens, a leader in digitalization for the manufacturing industries.
Click here for more information on intelligent design control in the medical device industry from our sponsor, Siemens, a leader in digitalization for the manufacturing industries.
https://youtu.be/z2zyO0O5o_Q
[post_title] => Digital Design Control for Medical Devices
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => med-dev-control
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:28:47
[post_modified_gmt] => 2022-11-15 03:28:47
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6700
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[5] => WP_Post Object
(
[ID] => 6645
[post_author] => 2
[post_date] => 2018-02-07 06:48:42
[post_date_gmt] => 2018-02-07 11:48:42
[post_content] => Medical Device manufacturers struggling with product complexity can leverage digitalization to more efficiently transfer product designs to the shop floor. This episode of Tech-Clarity TV shares how digitalization can combat long validation times and slow manufacturing ramp-up by creating a digital continuity between product designs and manufacturing processes. Digital design transfer brings the potential for:
Given the increased interest and adoption, it’s time to put together some selection criteria to help companies with their decisions. We recommend that companies pick their solution first and then choose their deployment option. This is effectively a “solution first” approach as opposed to a “cloud first” approach. We find that companies are just not willing to shortchange functionality in this crucial area. They recognize it’s important to evaluate the functional capabilities of a PLM system to ensure they’ll gain the significant top- and bottom-line benefits that PLM delivers.
There are still important decisions to make after a solution is selected. Many systems can be deployed in a variety of ways ranging from cloud Software as a Service (SaaS) to traditional, on-premise implementations. The deployment choice impacts important factors including cost, security, resource requirements, performance, risk, and time to benefit. This guide is designed to help companies navigate the options and choose the best-suited PLM cloud option for their business.
*This summary is an abbreviated version of the report and does not contain the full content. A link to download the full report is available above.
[post_title] => Cloud PLM Buyer's Guide
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-plm-guide
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:27:54
[post_modified_gmt] => 2022-11-15 03:27:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6708
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[4] => WP_Post Object
(
[ID] => 6700
[post_author] => 2
[post_date] => 2018-02-20 11:20:02
[post_date_gmt] => 2018-02-20 16:20:02
[post_content] => Medical Device companies have to manage complexity and regulation without placing excess overhead on their designers. Devices complexity has led to increased complaints, CAPAs, observations, and recalls. The root cause is frequently insufficient and cumbersome design control.
Digitalization helps them design with agility while maintaining control. It improves speed, accuracy, and productivity by automating submissions, DMRs, and DHFs from design data. The digital approach transitions device manufacturers away from document-centric and paper-based processes, or the electronic equivalent of processes that originated on paper that have been automated but never digitalized.
Digital design leverages complete digital models and creates a complete digital thread of information through design and the product lifecycle. It allows a cohesive approach where each step in design builds on the previous, and results in a digital model that engineers can use to validate and verify the behavior of the physical product in digital form using simulation.
Digital design control helps medical device companies get devices approved more quickly, keeps quality high, reduces risk, controls cost, and helps companies get innovative product to market quickly. The results are faster time to market, increased quality, and improved compliance.
This video series is sponsored by Siemens, a leader in digitalization for the manufacturing industries.
Click here for more information on intelligent design control in the medical device industry from our sponsor, Siemens, a leader in digitalization for the manufacturing industries.
https://youtu.be/z2zyO0O5o_Q
[post_title] => Digital Design Control for Medical Devices
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => med-dev-control
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:28:47
[post_modified_gmt] => 2022-11-15 03:28:47
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6700
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[5] => WP_Post Object
(
[ID] => 6645
[post_author] => 2
[post_date] => 2018-02-07 06:48:42
[post_date_gmt] => 2018-02-07 11:48:42
[post_content] => Medical Device manufacturers struggling with product complexity can leverage digitalization to more efficiently transfer product designs to the shop floor. This episode of Tech-Clarity TV shares how digitalization can combat long validation times and slow manufacturing ramp-up by creating a digital continuity between product designs and manufacturing processes. Digital design transfer brings the potential for:
- Simulating product, processes, lines, and plants
- Virtually commissioning production equipment
- Creating a seamless digital thread of information
- Transferring production process data to MES systems
 Jim Brown joins PTC's Alan Goldman and Mark Lobo for a web panel discussion on adopting Cloud PLM. Jim will share perspectives from his recent research including cloud PLM benefits and requirements. Jim will share insights from his upcoming Cloud PLM Buyer's guide including criteria companies should look for in a solution, for adoption, and their vendor partner. Jim and the other presenters will also anwer audience questions.
Jim, Alan, and Mark will be joined by Tim Curran of KPIT for a North American panel on Engineering360 on the same topic.
Register for the European webcast now (replay pending) (free of charge, registration required).
Register for the North American webinar now (free of charge, registration required)
[post_title] => Selecting the Right Cloud PLM System (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-plm-3
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6633
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[7] => WP_Post Object
(
[ID] => 6619
[post_author] => 2
[post_date] => 2018-01-31 15:32:51
[post_date_gmt] => 2018-01-31 20:32:51
[post_content] => Medical Device companies have the opportunity to leverage digitalization and the digital enterprise to develop innovative products and bring them to market quickly. Watch this edition of Tech-Clarity TV to learn about the digitalization opportunity and stay tuned for the rest of the series detailing specific business improvement opportunities including:
Jim Brown joins PTC's Alan Goldman and Mark Lobo for a web panel discussion on adopting Cloud PLM. Jim will share perspectives from his recent research including cloud PLM benefits and requirements. Jim will share insights from his upcoming Cloud PLM Buyer's guide including criteria companies should look for in a solution, for adoption, and their vendor partner. Jim and the other presenters will also anwer audience questions.
Jim, Alan, and Mark will be joined by Tim Curran of KPIT for a North American panel on Engineering360 on the same topic.
Register for the European webcast now (replay pending) (free of charge, registration required).
Register for the North American webinar now (free of charge, registration required)
[post_title] => Selecting the Right Cloud PLM System (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-plm-3
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6633
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[7] => WP_Post Object
(
[ID] => 6619
[post_author] => 2
[post_date] => 2018-01-31 15:32:51
[post_date_gmt] => 2018-01-31 20:32:51
[post_content] => Medical Device companies have the opportunity to leverage digitalization and the digital enterprise to develop innovative products and bring them to market quickly. Watch this edition of Tech-Clarity TV to learn about the digitalization opportunity and stay tuned for the rest of the series detailing specific business improvement opportunities including:
- Digital design
- Digital design transfer
- Paperless manufacturing
 Tech-Clarity's Medical Devices Manufacturers Software Selection Guide for 2018 helps manufacturers identify the right buying criteria for software solutions to support developing, producing, and servicing medical devices. For 2018, the guide has also been updated to include additional considerations for the Internet of Things (IoT). This buyer's guide also takes into account unique needs for medical device companies including regulatory compliance and support for the FDA's Case for Quality.
Tech-Clarity’s Buyer’s Guides go beyond software functionality to provide a framework of requirements that impact implementation success and long-term ROI, including:
Tech-Clarity's Medical Devices Manufacturers Software Selection Guide for 2018 helps manufacturers identify the right buying criteria for software solutions to support developing, producing, and servicing medical devices. For 2018, the guide has also been updated to include additional considerations for the Internet of Things (IoT). This buyer's guide also takes into account unique needs for medical device companies including regulatory compliance and support for the FDA's Case for Quality.
Tech-Clarity’s Buyer’s Guides go beyond software functionality to provide a framework of requirements that impact implementation success and long-term ROI, including:
- Software capabilities
- Implementation
- User adoption
- Support
- Vendor characteristics / attributes
- Unique business needs
Table of Contents
- Executive Overview
- Identify Your Challenges
- Transition from Document Centric to Product Centric
- Consider the Complete Lifecycle
- Manage the Product
- Manage Requirements (Customer Needs to Regulatory)
- Support Product Development for Hardware
- Support Software Development
- Enable Smart and Connected Products
- Ensure Regulatory Compliance
- Support Quality Management
- Plan for Manufacturing
- Control Suppliers
- Plan for Service
- Assess Implementation Requirements
- Consider Vendor Attributes
- Identify Specific Needs for your Company
- Conclusion
- Recommendations
- About the Author
Executive Overview
Medical device companies are in the business of making people’s lives better. As Joel Hembrock, Senior Designer and CAD Administrator at Medtronic says, “Our patients are the people who benefit from our products. Restoring life is our main focus. [We want] to be giving people their lives back, restoring their health, allowing them to actually live again and not have their disease or any other ailment keep them from being able to live.” It is an exciting time for the industry as technology advancements have opened up new and exciting opportunities that have the potential to further this mission. On top of this, an aging Baby Boomer population will create additional demand for medical devices. Consequently, the industry should see significant growth. In fact, the Evaluate MedTech World Preview 2017, Outlook to 2022 report forecasts a 5.1% growth rate every year for the next five years. However, it will be the companies who can overcome the unique challenges in the industry who will be best positioned to take advantage of this growth. With lives at stake, patient safety is of the utmost importance. As such, the industry faces heavy regulations. Compliance is so critical that if medical device companies do not adhere to FDA, EU and other worldwide standards and regulations, they will not be profitable. However, so much time, effort, and cost go into compliance documentation; it takes resources away from innovating and ensuring high quality products. As a result, it is harder to take advantage of opportunities that will boost profitability. On the other hand, medical device companies who adopt practices focused on high quality devices can expect greater patient satisfaction, improved competitiveness, and higher profits. In fact, McKinsey estimates that firms who embrace quality focused best practices can increase profits by 3% to 4% of revenues. They predict the revenue increase alone could be a $3.5 billion opportunity for the industry[1] and this doesn’t even factor in the profitability improvements of avoiding costly quality issues. Unfortunately, for the industry, quality has been getting worse, not better. The FDA’s Medical Device Recall Report FY 2003 to FY 2012 shows that there has been a striking 97% increase in the number of recalls. To reverse this trend, medical device companies should adopt new approaches that enable a greater focus on quality. One way to achieve this by shifting from a document centric process to one that is product centric. This shift enables more focus on high quality, innovative products that meet patient needs. The good news is that with investments in the right software solutions, this is possible. Software solutions can reduce manual, time intensive reporting processes to a push of a button. Rather than structuring processes around documentation, software solutions can allow you to focus on developing the right products and services that will meet patient needs. For these reasons, some medical device manufacturers integrate quality processes into their core product lifecycle management activities. By incorporating quality processes throughout the product design and delivery lifecycle, companies can improve efficiency. With this approach, instead of wasting efforts searching for compliance supporting documentation and reporting, companies can use that valuable time for can quality and innovation. The result will be higher profitability. Other changes in the medical device industry coming from recent trends such as the transition to outcome-based healthcare in the US. In some cases, to be compensated, medical professionals must show positive patient outcomes. A way to accomplish this is to take advantage of innovation enabled by the Internet of Things (IoT). For example, IoT combined with software capabilities can be used to meet requirements for Unique Device Identifiers (UDIs) that will provide new levels of traceability and communication to demonstrate device effectiveness. the right software solution must be in place to effectively manage it. While UDIs present one possible use case for IoT, the potential opportunities for IoT go even further. IoT and other technological advancements such as augmented reality (AR) and 3D printing, create new opportunities for profitable new business models by enabling services that align with customer and patient needs. However, companies stuck following traditional document centric workflows may struggle to find the bandwidth to innovate with these new technologies. Meanwhile, competitors who can devote the resources to innovation will be well positioned to capture a leading market position. This situation makes it an ideal time to invest in a software platform that enables a product centric approach for the entire lifecycle of your device. As you evaluate your current needs, think through how your business needs may evolve due to the impact of technological advances in medical devices. For example, designing for connectivity may require new approaches to design. You will now need to consider things like sensors, ecosystems, and new IT development roles that will play a critical part of product development. Ensure your software platform will be able to meet both current and future needs as your product portfolio evolves. While you consider investments in software solutions, leverage existing systems that are working well. The new solution should use a platform that will leverage and extend the investments made in existing solutions, such as Manufacturing Execution Systems (MES), Enterprise Resource Planning (ERP) and Product Lifecycle Management (PLM) if you are happy with them. With so much to consider, how do you know what will be the right software technology to support the lifecycle of your devices? This buyer’s guide will serve as guidance to help you select what is right for your company. This guide consists of four major sections covering software tool functionality required for medical device companies, implementation requirements, vendor attributes, and unique company considerations (Figure 1). Each section includes a checklist of key requirements to investigate when selecting software tools. This guide is not an all encompassing requirements list. It provides a high level overview. [1] Ted Fuhr, Katy George, Janice Pai, “The Business Case for Medical Device Quality,” McKinsey Center for Government, McKinsey & Company, October 2013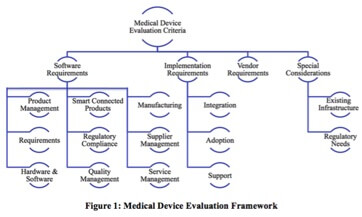
Conclusion
Medical device companies looking to improve profitability should shift their focus from a document centric process to a product centric process that will enable them to concentrate on quality. The cost of poor quality can be significant. In fact, McKinsey estimates that non-routine quality events cost the industry up to $5 billion. On the other hand, companies that focus on high quality enjoy a significant advantage with a potential increase in profits of 3% to 4% of revenues, according to McKinsey. The FDA has also concluded that a greater focus on quality is required. For example, they have found recalls have increased by 97%, with design errors being the leading cause. With the Case for Quality initiative, quality should be come an area of heightened focus, which a product centric approach supports. Unfortunately, making this shift, while still meeting regulatory requirements is hard. However, with the right technology, companies can make it much easier. The right solution should consider all aspects of the product lifecycle from requirements, through design, testing, manufacturing, and service. It should streamline the regulatory process and automate as much as possible. With traceability across the lifecycle and all deliverables, it will be much easier to provide regulatory compliance documentation. That traceability should also extend to suppliers. The right software solution can make it much easier to bring the right, high quality medical device to market, providing a competitive advantage. When making such a significant investment, you should also anticipate how your requirements will evolve to remain competitive in the future. Technological advancements such as IoT, AR, and 3D printing create opportunities for very profitable new business models that can increase the quality of patient care considerably. Features such as predictive and remote service offer flexibility around downtime to minimize the impact on patient health. These technologies can help medical device companies advance their mission of making people’s lives better.Recommendations
Based on industry experience and research for this report, Tech-Clarity offers the following recommendations:- Identify the top challenges your company needs to solve when bringing medical devices to market.
- Consider a solution that can support the entire lifecycle of your product, from patient needs and requirements to, design, test, manufacturing, and service.
- Look at solutions that will support the Case for Quality. A product centric approach and in integrated PLM and Quality Management System can help keep the focus on quality throughout the entire lifecycle.
- Use a vendor who is familiar with medical device regulatory requirements and has the technology to reduce the manual effort required to comply with regulations.
- Ensure you can manage the device and all associated document, design details, and changes while having traceability across everything.
- Support requirements with a solution that will work across all disciplines and has traceability across all stages and deliverables to support changes and compliance.
- Empower each team member including design, quality, procurement, manufacturing, and service with tools that work for them, while still ensuring a single source of truth for product information.
- Support quality management with traceability from requirements to test and reporting tools to ensure monitoring of trends that impact quality.
- Ensure tight controls on suppliers so as not to put compliance at risk.
- Select a solution that will support manufacturing so that you produce devices as designed and meet regulatory requirements.
- Think about medical device service requirements and use a solution that will support current and future service models such as predictive and remote service.
- Select a vendor who can integrate with your existing solutions while implementing new solutions where needed.
- Consider future needs for potential revenue streams and future needs for technologies such as IoT, 3D printing, and Augmented Reality.
Too Much Waste in Electrical Design
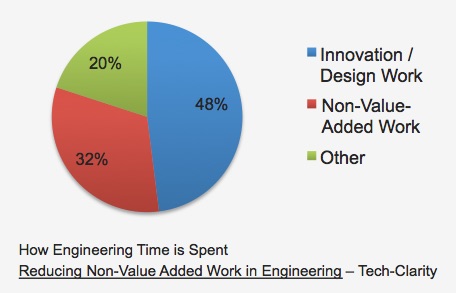 Engineers are the engines of product innovation. Their efforts turn product dreams and concepts into reality and, maybe most importantly, into company profits. Most companies recognize that their engineers need the right tools to get their work done. They enable them with a range of specialty tools for different aspects of design, analysis, and simulation. These are high-value solutions that help people design things that would be practically unachievable by more manual methods.
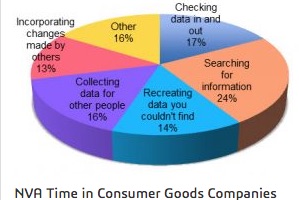
Why then, is there so much inefficiency in engineering? Our research, Reducing Non-Value-Added Work in Engineering, finds that technical resources spend about one-third of their time on non-valued-added (NVA) activity. The most significant portion of wasted time is spent simply searching for the information they need to do their jobs. For electrical engineering, too much time is lost searching for parts, looking up specs, waiting for data, reworking designs because of bad libraries, or resolving problems that other design teams already solved on an earlier design.
Beyond accessing the data they need, engineers spend a tremendous amount of time keeping work-in-progress (WIP) designs and processes under control. Or, perhaps worse, they waste time fixing problems resulting from poor control. These activities cost engineers, and their companies, significant time and money. More importantly, they slow the pace of innovation resulting in poor efficiency, excess cost, and late products. Engineers are constantly forced to take data management shortcuts in order to maintain schedule, mortgaging future gains in productivity to meet pressing deadlines.
Engineers are the engines of product innovation. Their efforts turn product dreams and concepts into reality and, maybe most importantly, into company profits. Most companies recognize that their engineers need the right tools to get their work done. They enable them with a range of specialty tools for different aspects of design, analysis, and simulation. These are high-value solutions that help people design things that would be practically unachievable by more manual methods.
Why then, is there so much inefficiency in engineering? Our research, Reducing Non-Value-Added Work in Engineering, finds that technical resources spend about one-third of their time on non-valued-added (NVA) activity. The most significant portion of wasted time is spent simply searching for the information they need to do their jobs. For electrical engineering, too much time is lost searching for parts, looking up specs, waiting for data, reworking designs because of bad libraries, or resolving problems that other design teams already solved on an earlier design.
Beyond accessing the data they need, engineers spend a tremendous amount of time keeping work-in-progress (WIP) designs and processes under control. Or, perhaps worse, they waste time fixing problems resulting from poor control. These activities cost engineers, and their companies, significant time and money. More importantly, they slow the pace of innovation resulting in poor efficiency, excess cost, and late products. Engineers are constantly forced to take data management shortcuts in order to maintain schedule, mortgaging future gains in productivity to meet pressing deadlines.
Conclusion
Today’s competitive global markets demand high levels of productivity. Over one-half of companies, however, suffer from design inefficiency due to data management challenges, according to our The Facts about Managing Product Data research. Companies can no long afford that level of NVA effort in engineering. It’s time to move away from informal methods that lead to errors and rework, and adopt disciplined methods to control, access, and share WIP electrical design data. This approach also helps engineers manage their processes and collaborate across electrical design disciplines. EDM is a critical element of the enterprise ecosystem. It integrates with PLM and ERP to provide the detailed WIP support needed for electrical designs in the context of an overall product innovation process. Electronic EDM is the missing element that gives electrical engineers the tools they need to be efficient and effective and get away from today’s high levels of NVA time. As a result, engineering can reduce non-value-added work, improve quality, reduce risk, and speed up design cycles and time to market.
Electrical EDM helps manufacturers:
EDM is a critical element of the enterprise ecosystem. It integrates with PLM and ERP to provide the detailed WIP support needed for electrical designs in the context of an overall product innovation process. Electronic EDM is the missing element that gives electrical engineers the tools they need to be efficient and effective and get away from today’s high levels of NVA time. As a result, engineering can reduce non-value-added work, improve quality, reduce risk, and speed up design cycles and time to market.
Electrical EDM helps manufacturers:
- Enable cross domain collaboration
- Drive real-time design and project visibility throughout the organization
- Manage WIP electrical design data without adding extra work steps
- Enable agility in electrical engineering workflows
- Efficiently find and reuse information when it’s needed
- Control, access, and share components
- Optimize technical and business needs via Strategic Parts Lists
- Control the engineering design, not just manage files
- Establish and implement best practice processes for design projects
How Managing Service Information Helps Your Business
In today’s highly competitive environment, it is hard to stand out. For many companies, providing customers with superior service gives them an edge in the market. To achieve higher levels of service, Top Performing companies combine best practices with technology to successfully manage service information. With the right technology, your business can:- Enable your technicians to find, understand and trust your product and parts information
- Reduce customer downtime by improving first time fix rates
- Increase service and technician efficiency
- Significantly lower overall service costs by reducing unnecessary repeat service visits
- Improve your brand reputation through superior service
 By ensuring technicians and customers have access to up-to-date service and parts information, you will increase customer satisfaction, leading to repeat business, a stronger brand reputation, and greater service revenue. The key is selecting the right solution. This Buyer’s Guide reveals what to look for in a service information management solution.
By ensuring technicians and customers have access to up-to-date service and parts information, you will increase customer satisfaction, leading to repeat business, a stronger brand reputation, and greater service revenue. The key is selecting the right solution. This Buyer’s Guide reveals what to look for in a service information management solution.
Select the Right Service Solution for your Needs
The right service information management solution can greatly improve service levels: Recommendations Invest in a solution that will improve customer satisfaction. This will lead to increased revenue growth.
Invest in a solution that will improve customer satisfaction. This will lead to increased revenue growth.- Focus on improving technician productivity by getting them the right information and parts at the right time.
- Leverage existing engineering data to produce service content.
- Ensure engineering and service information, including CAD models, remain linked so that information does not become outdated.
- Consider the needs of the extended enterprise and choose a solution that is compatible with existing solutions.
 Read the post (free of charge, no registration required).
[post_title] => Accelerating CPG Innovation with an Innovation Platform (guest post)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cpg-pip
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:35
[post_modified_gmt] => 2022-11-15 03:25:35
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6557
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[12] => WP_Post Object
(
[ID] => 6522
[post_author] => 2
[post_date] => 2017-12-21 15:27:51
[post_date_gmt] => 2017-12-21 20:27:51
[post_content] =>
Read the post (free of charge, no registration required).
[post_title] => Accelerating CPG Innovation with an Innovation Platform (guest post)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cpg-pip
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:35
[post_modified_gmt] => 2022-11-15 03:25:35
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6557
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[12] => WP_Post Object
(
[ID] => 6522
[post_author] => 2
[post_date] => 2017-12-21 15:27:51
[post_date_gmt] => 2017-12-21 20:27:51
[post_content] =>  Top 5 Ways to Measure Product Innovation, Choosing Metrics to Drive Innovation Performance shares the importance of measuring innovation to improve innovation capability and outcomes.
Please enjoy the summary below, or click the report to download a PDF overview (free of charge, no registration required).
For the full report, please visit our sponsor, Planview (free of charge, registration required).
Top 5 Ways to Measure Product Innovation, Choosing Metrics to Drive Innovation Performance shares the importance of measuring innovation to improve innovation capability and outcomes.
Please enjoy the summary below, or click the report to download a PDF overview (free of charge, no registration required).
For the full report, please visit our sponsor, Planview (free of charge, registration required).
Why Measure Innovation?
Companies know they’ll be rewarded for innovation. They’ve seen that product developers who set the agenda in their market and force their competitors to react have a distinct advantage. But how do they know if they’re being innovative before they get invited to accept their industry’s “top innovator” award? One of the most common questions we’re asked is “how should we measure innovation?” It’s a relatively simple question, but one that doesn’t come with a simple answer. There’s no “silver bullet” way to measure it. First of all, innovation means different things by industry and by company. It depends on your business strategy and how you choose to compete. For example, measures would be different for a goal of true market disruption versus more incremental innovation. Innovation also has different levels of granularity. It may apply to a product line, a product, or a feature – each with different impacts and potentially different ways to measure success. This is without even considering organizations who call process improvements, safety initiatives, and lean measures “innovation,” which can further complicate things. With all of the potential variability, let’s step back and talk about why it’s important to measure innovation in the first place. Metrics help drive desired outcomes. We find that companies want to:- Make innovation as systematic as possible
- Have a repeatable innovation capability
- Drive the right organizational behavior
- Achieve more reliable and predictable outcomes
- Improve innovation performance over time
Table of Contents
- Why Measure Innovation?
- Measuring by Outcomes
- Measuring Innovation Capability
- Measuring Innovation Contributions
- Measuring the Strategic Portfolio
- Measuring Calculated Risk Taking
- Measuring Time and Space to Innovate
- Measuring NPDI Control
- What’s Needed to Measure Innovation?
- Conclusion
- About the Author
Conclusion
Innovation is clearly important to company success, and what gets measured gets improved. This makes measuring innovation an important goal. It’s also a challenging one. There are a number of ways that companies can measure innovation performance by outcomes, such as the percentage of revenue from new products, but this information typically comes too late to make in-course adjustments and improvements. Another approach is to introduce measures that ensure the environment is conducive to innovation. Companies can put in place measurements that help ensure the organization is contributing to innovation and product portfolios contain enough innovation of the right variety. Organizations can survey whether employees feel they have the ability to take calculated risks and whether they have the time and space required to innovate. Metrics can also ensure that their processes and tools allow them to profit from innovation through effective NPDI. For any of these metrics to be effective, companies need to have the right process and data management infrastructure in place, including:
For any of these metrics to be effective, companies need to have the right process and data management infrastructure in place, including:
- The right data and a mechanism to gather it efficiently
- Accurate, visual reporting tools and dashboards that they can drill down from
- Honest information and the ability to capture knowledge – especially failures – to drive future innovation
 Tech-Clarity’s BOM Management Buyer’s Guide - Boost Performance with Digital BOMs provides criteria for manufacturers to evaluate software solutions to support their Bill of Material and product structure data and processes. Tech-Clarity’s Buyer’s Guides go beyond software functionality to provide a framework of requirements that impact implementation success and long-term ROI, including:
Tech-Clarity’s BOM Management Buyer’s Guide - Boost Performance with Digital BOMs provides criteria for manufacturers to evaluate software solutions to support their Bill of Material and product structure data and processes. Tech-Clarity’s Buyer’s Guides go beyond software functionality to provide a framework of requirements that impact implementation success and long-term ROI, including:
- Software requirements
- Implementation
- Integration
- User adoption
- Support
- Vendor characteristics / attributes
- Industry or unique business needs
Executive Overview
Managing Bills of Material (BOMs) is a fundamental need for any manufacturer. Without effective control of product structures, companies struggle with inefficiency and errors. On the other hand, improving the maturity of BOM-related processes helps to manage complexity, improve efficiency, prevent mistakes, and enhance collaboration across departments and the supply chain. The resulting benefits can be strategic, leading to increased agility and faster time to market that impact top-line financial performance. Beyond improving today’s performance, driving better BOM management creates the foundation for even greater improvements as a step toward the digital enterprise.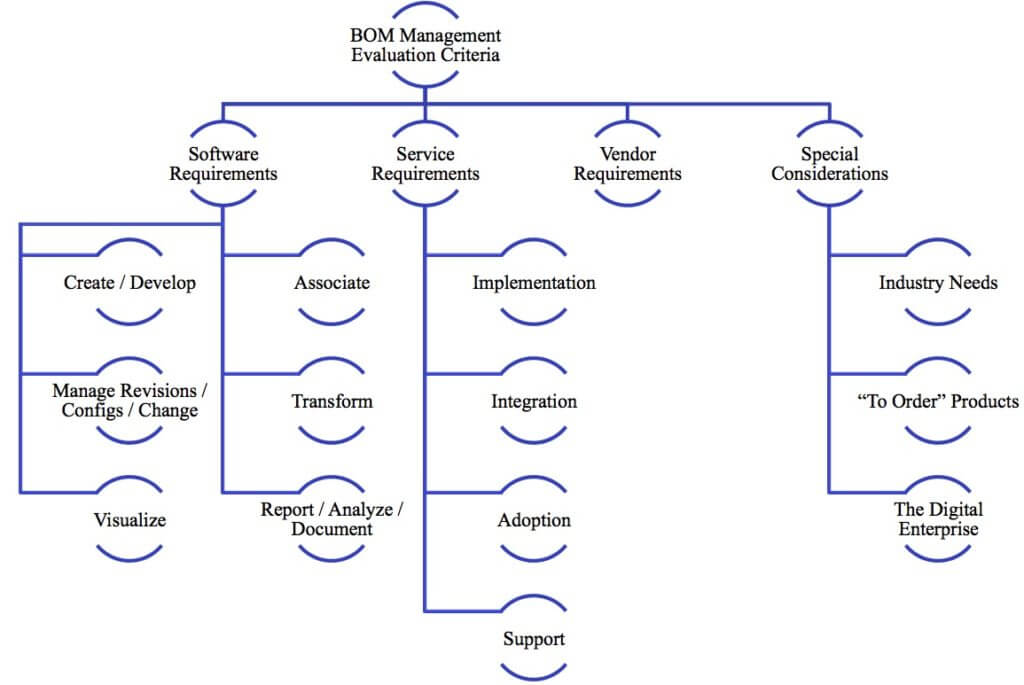 This Buyer’s Guide is a reference tool for manufacturers selecting a system to improve the maturity of their BOM management practices. The guide is composed of sections covering software, service, and vendor requirements plus some special considerations. These are all important factors that impact implementation success and ROI.
Each of these sections includes a checklist with key requirements to investigate when selecting software to enable and improve BOM management. The guide also touches on special considerations for companies with “to order” products and a few special considerations to keep an eye on by industry. It also touches on the importance of improving BOM management to support digital enterprise opportunities including the Internet of Things (IoT), Virtual Reality (VR), and Augmented Reality (AR).
This Buyer’s Guide is a reference tool for manufacturers selecting a system to improve the maturity of their BOM management practices. The guide is composed of sections covering software, service, and vendor requirements plus some special considerations. These are all important factors that impact implementation success and ROI.
Each of these sections includes a checklist with key requirements to investigate when selecting software to enable and improve BOM management. The guide also touches on special considerations for companies with “to order” products and a few special considerations to keep an eye on by industry. It also touches on the importance of improving BOM management to support digital enterprise opportunities including the Internet of Things (IoT), Virtual Reality (VR), and Augmented Reality (AR).
Table of Contents
- Executive Overview
- Diagnosing BOM Management Issues
- The BOM Management Status Quo
- The BOM Management Business Case
- Analyze BOM Management Solution Capabilities
- Assess Service Requirements
- Consider Vendor Requirements
- Special Considerations
- Conclusion
- Recommendations
- About the Author
Conclusion
BOM management helps manage complexity and streamlines operations. It provides an important, foundational element that serves as the backbone for all engineering, manufacturing, and service activity. An accessible, trusted source of product structure information is valuable and improves traceability and control. “Our objective was to create a single, standardized global product structure and we’ve achieved that. Any designer at any time can collaborate and participate with a common dataset,” says Mark Mitchell of Jabil. “People have the information they need, and nobody needs to call me during an audit – that’s the best metric!” he concludes. Effective BOM management provides enterprise-level benefits, improving business performance and alleviating disconnects across the business. “PLM is not about optimizing within silos, it’s about connecting across silos. BOM management helps us streamline and prevent errors across the product lifecycle,” offers a PLM Architect. The net result is efficiency and cost gains combined with revenue improvement from better collaboration and faster time to market, making BOM management and important operational tool and a key driver of improved profitability. Supporting BOM management at the enterprise level requires the right solution. It’s important to evaluate key solution characteristics, but also to go beyond. Companies should develop a requirements list that helps encourage a holistic decision encompassing software functionality, service-related needs, vendor requirements, and any special considerations based on their industry, size, and product strategy. Finally, the plan should look beyond current needs to support the digital future where digital twins, AR, VR, and IoT rely on sound BOM information.Recommendations
Based on industry experience and research for this report, Tech-Clarity offers the following recommendations:- Think big, but remain agile and take BOM management improvements in steps
- Recognize the importance of accurate, complete, timely, and accessible product structures
- Know your needs
- Understand the value
- Look for functionality, but extend requirements to vendor and service
- Consider any special needs for your business, industry, or geography
- Build the foundation for the digital enterprise, recognizing the BOM management is a key enabler
- Get started
 Register for the December 13 webcast (free of charge, registration required). Sponsored by Dassault Systemes BIOVIA.
[post_title] => Digitalizing the Chemical Lab (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => digital-lab
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6496
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[17] => WP_Post Object
(
[ID] => 6478
[post_author] => 2
[post_date] => 2017-12-04 10:58:06
[post_date_gmt] => 2017-12-04 15:58:06
[post_content] => This Tech-Clarity TV animation explains how companies can combat industry disruption from new, innovative companies and business models in the food and beverage industry by adopting the digital enterprise. It's no secret that companies like Amazon, Blue Apron, HelloFresh, and more are changing the way consumers and consumer packaged goods companies relate. This video shares how companies can become more innovative and agile to compete with these challengers through the value of digitalization, and provides an example of what a digital food and beverage company looks like.
https://youtu.be/9eWSdupjl3Y
The video series is sponsored by Siemens, a leader in digitalization. For more information on digitalization in the food and beverage industry, please see our series of blog posts on digitalization in the food and beverage industry on the Siemens blog and related video on Raising the Bar in R&D with Digitalization.
[post_title] => Digitalization in the Food and Beverage Industry (Animation)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => dig-fb-animation
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:05:49
[post_modified_gmt] => 2022-12-02 20:05:49
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6478
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[18] => WP_Post Object
(
[ID] => 6466
[post_author] => 2
[post_date] => 2017-11-29 16:24:42
[post_date_gmt] => 2017-11-29 21:24:42
[post_content] => Join Tech-Clarity's Jim Brown in a lively discussion with Planview NPD Evangelist Carrie Nauyalis discussing how to effectively measure product innovation. The duo will discuss the pitfalls of measuring innovation based on prior performance, the top five measures companies can use to measure innovation capability, and what's needed to get started measuring innovation to improve outcomes.
Register for the December 13 webcast (free of charge, registration required). Sponsored by Dassault Systemes BIOVIA.
[post_title] => Digitalizing the Chemical Lab (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => digital-lab
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6496
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[17] => WP_Post Object
(
[ID] => 6478
[post_author] => 2
[post_date] => 2017-12-04 10:58:06
[post_date_gmt] => 2017-12-04 15:58:06
[post_content] => This Tech-Clarity TV animation explains how companies can combat industry disruption from new, innovative companies and business models in the food and beverage industry by adopting the digital enterprise. It's no secret that companies like Amazon, Blue Apron, HelloFresh, and more are changing the way consumers and consumer packaged goods companies relate. This video shares how companies can become more innovative and agile to compete with these challengers through the value of digitalization, and provides an example of what a digital food and beverage company looks like.
https://youtu.be/9eWSdupjl3Y
The video series is sponsored by Siemens, a leader in digitalization. For more information on digitalization in the food and beverage industry, please see our series of blog posts on digitalization in the food and beverage industry on the Siemens blog and related video on Raising the Bar in R&D with Digitalization.
[post_title] => Digitalization in the Food and Beverage Industry (Animation)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => dig-fb-animation
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:05:49
[post_modified_gmt] => 2022-12-02 20:05:49
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6478
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[18] => WP_Post Object
(
[ID] => 6466
[post_author] => 2
[post_date] => 2017-11-29 16:24:42
[post_date_gmt] => 2017-11-29 21:24:42
[post_content] => Join Tech-Clarity's Jim Brown in a lively discussion with Planview NPD Evangelist Carrie Nauyalis discussing how to effectively measure product innovation. The duo will discuss the pitfalls of measuring innovation based on prior performance, the top five measures companies can use to measure innovation capability, and what's needed to get started measuring innovation to improve outcomes.
 Register for the November 30th webcast now, sponsored by Planview (free of charge, registration required).
[post_title] => Top 5 Metrics to Stop Measuring Innovation in the Rearview Mirror (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => innovation-metrics
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:53
[post_modified_gmt] => 2022-11-15 03:26:53
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6466
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[19] => WP_Post Object
(
[ID] => 6458
[post_author] => 2
[post_date] => 2017-11-27 09:54:34
[post_date_gmt] => 2017-11-27 14:54:34
[post_content] => Jim Brown will moderate this IEEE webcast featuring Gentherm Director of Engineering Systems Marinko Lazanja and PTC's Senior Director of Product Management Graham Birch. The webcast will share the importance of managing Bills of Material and product structures to avoid common inefficiencies and errors, but also to serve as the digital product backbone for the digital enterprise. The webinar will be an interactive discussion focusing on the things companies should look for when evaluating a solution to support BOM Management, including:
Register for the November 30th webcast now, sponsored by Planview (free of charge, registration required).
[post_title] => Top 5 Metrics to Stop Measuring Innovation in the Rearview Mirror (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => innovation-metrics
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:53
[post_modified_gmt] => 2022-11-15 03:26:53
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6466
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[19] => WP_Post Object
(
[ID] => 6458
[post_author] => 2
[post_date] => 2017-11-27 09:54:34
[post_date_gmt] => 2017-11-27 14:54:34
[post_content] => Jim Brown will moderate this IEEE webcast featuring Gentherm Director of Engineering Systems Marinko Lazanja and PTC's Senior Director of Product Management Graham Birch. The webcast will share the importance of managing Bills of Material and product structures to avoid common inefficiencies and errors, but also to serve as the digital product backbone for the digital enterprise. The webinar will be an interactive discussion focusing on the things companies should look for when evaluating a solution to support BOM Management, including:
- Software Requirements (functionality)
- Service Needs (including adoption and training)
- Vendor Attributes
- Special Considerations
 You can also register for a November 28 European webcast at 8:00 AM Eastern featuring PDM Vision Group's Jonas Härdner, Graham Birch, and Jim Brown.
This webcast is free of charge, registration required. Sponsored by PTC.
[post_title] => Select the Right PLM Solution for BOM Management (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => bom-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:53
[post_modified_gmt] => 2022-11-15 03:26:53
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6458
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
)
[post_count] => 20
[current_post] => -1
[before_loop] => 1
[in_the_loop] =>
[post] => WP_Post Object
(
[ID] => 6740
[post_author] => 2572
[post_date] => 2018-03-07 11:30:20
[post_date_gmt] => 2018-03-07 16:30:20
[post_content] =>
You can also register for a November 28 European webcast at 8:00 AM Eastern featuring PDM Vision Group's Jonas Härdner, Graham Birch, and Jim Brown.
This webcast is free of charge, registration required. Sponsored by PTC.
[post_title] => Select the Right PLM Solution for BOM Management (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => bom-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:53
[post_modified_gmt] => 2022-11-15 03:26:53
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://tech-clarity.com/?p=6458
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
)
[post_count] => 20
[current_post] => -1
[before_loop] => 1
[in_the_loop] =>
[post] => WP_Post Object
(
[ID] => 6740
[post_author] => 2572
[post_date] => 2018-03-07 11:30:20
[post_date_gmt] => 2018-03-07 16:30:20
[post_content] =>  Does having multiple systems to manage product information create challenges for your company? Do you find some systems are so heavily customized, it's hard to take advantage of the latest software enhancements? How can a PLM medical device solution help? If you have thought about any of these questions, you will find this webinar especially interesting, especially if you are in the medical device industry.
Does having multiple systems to manage product information create challenges for your company? Do you find some systems are so heavily customized, it's hard to take advantage of the latest software enhancements? How can a PLM medical device solution help? If you have thought about any of these questions, you will find this webinar especially interesting, especially if you are in the medical device industry.
Listen to Tina Kunshier of Boston Scientific and Tech-Clarity's Michelle Boucher discuss how to select the right software solution to support the development of medical devices. This discussion will be a live interview highlighting topics such as:
- The PLM journey taken by Boston Scientific
- What drove Boston Scientific to consolidate PLM systems
- How Boston Scientific executed their strategy for a unified PLM system
- Selection criteria you should look for in a PLM solution, especially for medical device companies
- Advice to improve product development efficiencies and support regulatory compliance
 [post_title] => How Boston Scientific Selected their PLM Medical Device Software Solution (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => boston-scientific-plm-medical-device
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6740
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[comment_count] => 0
[current_comment] => -1
[found_posts] => 882
[max_num_pages] => 45
[max_num_comment_pages] => 0
[is_single] =>
[is_preview] =>
[is_page] =>
[is_archive] =>
[is_date] =>
[is_year] =>
[is_month] =>
[is_day] =>
[is_time] =>
[is_author] =>
[is_category] =>
[is_tag] =>
[is_tax] =>
[is_search] =>
[is_feed] =>
[is_comment_feed] =>
[is_trackback] =>
[is_home] => 1
[is_privacy_policy] =>
[is_404] =>
[is_embed] =>
[is_paged] =>
[is_admin] =>
[is_attachment] =>
[is_singular] =>
[is_robots] =>
[is_favicon] =>
[is_posts_page] =>
[is_post_type_archive] =>
[query_vars_hash:WP_Query:private] => 459edbdc7ebea6a8c6645bb2c43289f2
[query_vars_changed:WP_Query:private] => 1
[thumbnails_cached] =>
[allow_query_attachment_by_filename:protected] =>
[stopwords:WP_Query:private] =>
[compat_fields:WP_Query:private] => Array
(
[0] => query_vars_hash
[1] => query_vars_changed
)
[compat_methods:WP_Query:private] => Array
(
[0] => init_query_flags
[1] => parse_tax_query
)
[query_cache_key:WP_Query:private] => wp_query:3264184e05005014fc7a90cc4bbcfcc7:0.83714700 17722267480.84631300 1772226748
)
[post_title] => How Boston Scientific Selected their PLM Medical Device Software Solution (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => boston-scientific-plm-medical-device
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:54
[post_modified_gmt] => 2022-11-15 03:26:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=6740
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[comment_count] => 0
[current_comment] => -1
[found_posts] => 882
[max_num_pages] => 45
[max_num_comment_pages] => 0
[is_single] =>
[is_preview] =>
[is_page] =>
[is_archive] =>
[is_date] =>
[is_year] =>
[is_month] =>
[is_day] =>
[is_time] =>
[is_author] =>
[is_category] =>
[is_tag] =>
[is_tax] =>
[is_search] =>
[is_feed] =>
[is_comment_feed] =>
[is_trackback] =>
[is_home] => 1
[is_privacy_policy] =>
[is_404] =>
[is_embed] =>
[is_paged] =>
[is_admin] =>
[is_attachment] =>
[is_singular] =>
[is_robots] =>
[is_favicon] =>
[is_posts_page] =>
[is_post_type_archive] =>
[query_vars_hash:WP_Query:private] => 459edbdc7ebea6a8c6645bb2c43289f2
[query_vars_changed:WP_Query:private] => 1
[thumbnails_cached] =>
[allow_query_attachment_by_filename:protected] =>
[stopwords:WP_Query:private] =>
[compat_fields:WP_Query:private] => Array
(
[0] => query_vars_hash
[1] => query_vars_changed
)
[compat_methods:WP_Query:private] => Array
(
[0] => init_query_flags
[1] => parse_tax_query
)
[query_cache_key:WP_Query:private] => wp_query:3264184e05005014fc7a90cc4bbcfcc7:0.83714700 17722267480.84631300 1772226748
)
All Results for "All"
Service Information: The Pivotal Factor in Your Aftermarket Business (webcast)
Listen to, PTC’s Dave Duncan and Tech-Clarity’s Michelle Boucher discuss how to improve the accuracy of service information for field personnel. They share stories about Embraer, Airbus Helicopters, and Kirloskar Oil Engines and explain how they are transforming their service organizations. During this webcast, you will also learn: How poor service information affects technician productivity, customer satisfaction,…
Digitalization in the Medical Device Industry (animation)
How can digitalization help medical device companies improve innovation and drive profitable growth despite increased complexity and regulatory scrutiny? This animated video offers a look at what a digital medical device company looks like and offers three initiatives companies can adopt to start their digital transformation. Digitalization offers significant benefits to medical device manufacturers because…
Cloud PLM Buyer’s Guide
What should manufacturers look for when they buy cloud software for Product Lifecycle Management? Our PLM Cloud Buyer’s Guide shares the important factors companies need to know when they evaluate cloud solutions. The guide explains the reasons companies are pursuing cloud options including standard benefits like reduced implementation cost and faster time to benefit, but…
Digital Design Control for Medical Devices
Medical Device companies have to manage complexity and regulation without placing excess overhead on their designers. Devices complexity has led to increased complaints, CAPAs, observations, and recalls. The root cause is frequently insufficient and cumbersome design control. Digitalization helps them design with agility while maintaining control. It improves speed, accuracy, and productivity by automating submissions,…
Digital Design Transfer for Medical Devices (video)
Medical Device manufacturers struggling with product complexity can leverage digitalization to more efficiently transfer product designs to the shop floor. This episode of Tech-Clarity TV shares how digitalization can combat long validation times and slow manufacturing ramp-up by creating a digital continuity between product designs and manufacturing processes. Digital design transfer brings the potential for:…
Selecting the Right Cloud PLM System (webcast)
Jim Brown joins PTC’s Alan Goldman and Mark Lobo for a web panel discussion on adopting Cloud PLM. Jim will share perspectives from his recent research including cloud PLM benefits and requirements. Jim will share insights from his upcoming Cloud PLM Buyer’s guide including criteria companies should look for in a solution, for adoption, and their vendor partner….
The Digitalization Opportunity for Medical Device Companies (video)
Medical Device companies have the opportunity to leverage digitalization and the digital enterprise to develop innovative products and bring them to market quickly. Watch this edition of Tech-Clarity TV to learn about the digitalization opportunity and stay tuned for the rest of the series detailing specific business improvement opportunities including: Digital design Digital design transfer…
Medical Devices Manufacturers Software Selection Guide for 2018
Tech-Clarity’s Medical Devices Manufacturers Software Selection Guide for 2018 helps manufacturers identify the right buying criteria for software solutions to support developing, producing, and servicing medical devices. For 2018, the guide has also been updated to include additional considerations for the Internet of Things (IoT). This buyer’s guide also takes into account unique needs for medical device…
Managing Electronic Design Data and WIP (eBook)
The Managing Work in Progress in Electrical Design ebook shares best practices for managing electrical engineering data, designs, and processes. The research explains the role that Electronic Data Management (EDM) plays alongside other solutions including PDM, ALM, and PLM that manage mechanical CAD, software development, and the overall product development process respectively. EDM plays a…
Buyer’s Guide for Managing Service Information (eBook, survey results)
Tech-Clarity’s Buyer’s Guide for Managing Service Information identifies the biggest challenges of managing service and parts information. It then looks at how Top Performing companies overcome these companies and what they look for in a software solution to support them. One of the top things they do is leverage the information produced by engineering to support…
Accelerating CPG Innovation with an Innovation Platform (guest post)
Jim Brown contributed his thoughts on Accelerating CPG Innovation with an Innovation Platform to the Dassault Systèmes 3D Perspectives Blog. He shares his views on the innovation imperative in CPG and how consumer goods companies can take innovation to the next level using a modern Product Innovation Platform (PIP). The post explains how an effective…
Top 5 Ways to Measure Product Innovation (white paper)
Top 5 Ways to Measure Product Innovation, Choosing Metrics to Drive Innovation Performance shares the importance of measuring innovation to improve innovation capability and outcomes. Please enjoy the summary below, or click the report to download a PDF overview (free of charge, no registration required). For the full report, please visit our sponsor, Planview (free…
The Analytics Opportunity for Food and Beverage in the Digital Age (video)
This episode of Tech-Clarity TV explains how food and beverage companies can survive in the digital age as new, agile, innovative companies disrupt their markets. It shares how these food and CPG companies can use analytics as a competitive tool to better sense and respond to customer needs and gain deeper understanding of their products, processes,…
BOM Management Buyer’s Guide
Tech-Clarity’s BOM Management Buyer’s Guide – Boost Performance with Digital BOMs provides criteria for manufacturers to evaluate software solutions to support their Bill of Material and product structure data and processes. Tech-Clarity’s Buyer’s Guides go beyond software functionality to provide a framework of requirements that impact implementation success and long-term ROI, including: Software requirements Implementation Integration User…
Producing Food and Beverages in the Digital Age (video)
This episode of Tech-Clarity TV explains how digital food and beverage companies improve agility, innovation, and productivity through better connectivity between R&D and the plant, streamlined production operations, and analytics. This video is part of a series of videos showing how digitalization can help food and beverage companies compete with innovative, digital industry competitors that…
Digitalizing the Chemical Lab (webcast)
How do chemical companies apply digital to the chem lab? Digitalization is streamlining the way companies innovate and bring products to market. Tech-Clarity’s Jim Brown will share findings from his research on how chemical companies leverage the digital enterprise to improve efficiency and reduce cost while dealing with mounting regulatory and sustainability pressure. Register for…
Digitalization in the Food and Beverage Industry (Animation)
This Tech-Clarity TV animation explains how companies can combat industry disruption from new, innovative companies and business models in the food and beverage industry by adopting the digital enterprise. It’s no secret that companies like Amazon, Blue Apron, HelloFresh, and more are changing the way consumers and consumer packaged goods companies relate. This video shares how…
Top 5 Metrics to Stop Measuring Innovation in the Rearview Mirror (webcast)
Join Tech-Clarity’s Jim Brown in a lively discussion with Planview NPD Evangelist Carrie Nauyalis discussing how to effectively measure product innovation. The duo will discuss the pitfalls of measuring innovation based on prior performance, the top five measures companies can use to measure innovation capability, and what’s needed to get started measuring innovation to improve outcomes….
Select the Right PLM Solution for BOM Management (webcast)
Jim Brown will moderate this IEEE webcast featuring Gentherm Director of Engineering Systems Marinko Lazanja and PTC’s Senior Director of Product Management Graham Birch. The webcast will share the importance of managing Bills of Material and product structures to avoid common inefficiencies and errors, but also to serve as the digital product backbone for the…