How can manufacturers maximize value from their digital transformation efforts in 2021? In these uncertain times, it matters. There are some basics to leveraging Industry 4.0 trends. Success may be closer to your grasp than you realize. This live webinar has passed, but please click here to watch it free, on-demand. Find out where trends…
- Agility and flexibility: Can you handle uncertainty and respond as the market changes? This is at the heart of Industry 4.0. It's also crucial to navigating the effects of COVID-19.
- Build from a strong foundation: Will your new technology projects fail? Discover why they are doomed unless you have strong enterprise systems as underpinning.
- Smooth data flows: Do your people always have a clear source of the truth? Why master data management is essential to speed and agility of response.
- New business models: Can your company stay profitable and competitive in 2021? How manufacturers can move toward added-value services, improving sustainability, and new channels to market.
 Tech-Clarity’s eBook, What’s the Cost of Poor Collaboration? examines this topic for product development, sharing survey results from 155 manufacturers. Collaboration impacts every part of product development, and products cannot be developed or brought to market without it. While collaboration can be abstract and hard to measure, it's impact can be significant, especially when it is poor. The research reveals how it impacts your product design and development processes. It also identifies six areas of opportunity for collaboration improvements that can boost product profitability for your company.
Please enjoy the summary* below. For the full research, please visit our sponsor, Dassault Systèmes SOLIDWORKS (registration required).
Tech-Clarity’s eBook, What’s the Cost of Poor Collaboration? examines this topic for product development, sharing survey results from 155 manufacturers. Collaboration impacts every part of product development, and products cannot be developed or brought to market without it. While collaboration can be abstract and hard to measure, it's impact can be significant, especially when it is poor. The research reveals how it impacts your product design and development processes. It also identifies six areas of opportunity for collaboration improvements that can boost product profitability for your company.
Please enjoy the summary* below. For the full research, please visit our sponsor, Dassault Systèmes SOLIDWORKS (registration required).
Table of Contents
- Executive Summary
- What's Most Important for Design Team Success
- The Cost of Poor Collaboration on Engineers
- The Cost of Poor Collaboration on the Business
- Why Collaboration is So Critical
- Collaboration Requirements Have Increased
- Identifying Best Practices
- 1. Improve Engineering Efficiency
- 2. Recognize Collaboration Requirements
- 3. Provide Non-CAD Users Visibility to CAD
- 4. Improve Engineering / Manufacturing Collaboration
- 5. Connect Engineers and Simulation Analysts throughout the Design Process
- 6. Support Market Validation with Improved Customer Collaboration
- Recommendations
- About the Research
- Acknowledgments
Executive Summary
Collaboration Impacts Engineering Efficiency Survey results show that engineering efficiency is the top goal for product development success. Effective collaboration is critical for improving efficiency, yet many companies struggle with it, while others don't recognize the underlying connection between the two.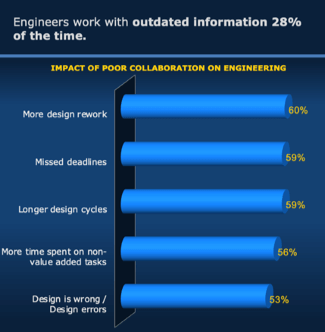 While poor collaboration is not a new problem, its cost has never been higher. Today's complex products and
the ecosystems we develop them in have raised collaboration needs so much, 40% of engineering time is now directly impacted by their ability to work together. With this much time affected, poor collaboration can cost companies significantly. While many companies struggle with steep competition, shrinking margins, and uncertain economic times, this is a risk few can afford.
Poor Collaboration has a Business Cost
Unfortunately, poor collaboration is so common, engineers report they work with outdated data 28% of the time. This results in more rework, delays, and errors. These negative business impacts mean lower-quality products, higher costs, missed deadlines, and delays in time to market.
In fact, what has traditionally worked in the past is no longer enough to stay competitive in today's market. An overwhelming 93% of companies report they need to improve collaboration with different groups. On average, engineers say they collaborate with 21 people on simple products and 35 for more complex products. Collaborators include other engineers, manufacturing, suppliers, customers, product managers, and more. On top of all the other design responsibilities, that's a lot of people to manage, work to keep track of, and risk for errors, without helpful solutions in place. No wonder the cost of poor collaboration is so high!
New Opportunities for Solutions
Many companies just live with their collaboration challenges, but as collaboration needs increase, these problems become harder and harder to ignore. As new technologies, such as the cloud and innovation platforms, break down silos and collaboration barriers, companies can benefit from new approaches to solve these challenges. Those who do should gain a competitive advantage.
While poor collaboration is not a new problem, its cost has never been higher. Today's complex products and
the ecosystems we develop them in have raised collaboration needs so much, 40% of engineering time is now directly impacted by their ability to work together. With this much time affected, poor collaboration can cost companies significantly. While many companies struggle with steep competition, shrinking margins, and uncertain economic times, this is a risk few can afford.
Poor Collaboration has a Business Cost
Unfortunately, poor collaboration is so common, engineers report they work with outdated data 28% of the time. This results in more rework, delays, and errors. These negative business impacts mean lower-quality products, higher costs, missed deadlines, and delays in time to market.
In fact, what has traditionally worked in the past is no longer enough to stay competitive in today's market. An overwhelming 93% of companies report they need to improve collaboration with different groups. On average, engineers say they collaborate with 21 people on simple products and 35 for more complex products. Collaborators include other engineers, manufacturing, suppliers, customers, product managers, and more. On top of all the other design responsibilities, that's a lot of people to manage, work to keep track of, and risk for errors, without helpful solutions in place. No wonder the cost of poor collaboration is so high!
New Opportunities for Solutions
Many companies just live with their collaboration challenges, but as collaboration needs increase, these problems become harder and harder to ignore. As new technologies, such as the cloud and innovation platforms, break down silos and collaboration barriers, companies can benefit from new approaches to solve these challenges. Those who do should gain a competitive advantage.
Recommendations
Next Steps Based on industry experience and research for this report, Tech-Clarity offers the following recommendations:- Understand the true cost of poor collaboration on both engineers and the entire company.
- Invest in collaboration improvements to increase engineering efficiency.
- Recognize the significance of collaboration requirements on engineers from the number of people involved, different departments, and processes impacted.
- Do not overlook the importance of engineering collaboration with non-CAD users.
- Ensure excellent collaboration between engineering and manufacturing to overcome knowledge gaps and support seamless hand-offs.
- Support effective collaboration between design engineers and simulation analysts to empower engineers to catch problems and design more competitive products.
- Incorporate customer collaboration into product development processes to support ongoing market validation and reduce the risk around market uncertainty.
- Consider modern technologies, such as cloud and an innovation platform, to support and enable better collaboration processes.
 How are companies adopting design and engineering solutions on the cloud?
Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives and examples.
[audio mp3="https://tech-clarity.com/wp-content/uploads/2020/10/Design-and-Engineer-Cloud-Adoption-2020-10-06-NEW.mp3"][/audio]
How are companies adopting design and engineering solutions on the cloud?
Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives and examples.
[audio mp3="https://tech-clarity.com/wp-content/uploads/2020/10/Design-and-Engineer-Cloud-Adoption-2020-10-06-NEW.mp3"][/audio]
 You can also see related video interviews including: Engineering in the Cloud Conversation and Designing on the Cloud Discussion with Paul Brown, Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell and Siemens Digital Transformation Progress with Brenda Discher.
Transcript:
Jim: Hi, I'm Jim Brown, President of Tech-Clarity, where we make the business value of technology clear. Today, I'm joined again by Paul Brown, Senior Marketing Director at Siemens Digital Industries Software. Paul's been very generous with his time. We've had a chance on this podcast to discuss design solutions moving to the cloud, things like CAD, but also a broader conversation about engineering software moving to the cloud, including things like visualization, rendering, simulation, and a little bit broader conversation about digital transformation. Now I want to really ask Paul, how do engineers adopt these solutions? How do engineers adopt these solutions? Let's get some clarity.
Paul, welcome, always a pleasure, I appreciate you coming back onto the podcast.
Paul: It's great to be here, Jim.
Jim: Paul, I know that you get a chance to speak with customers, and I know we're probably in more virtual conversations today than we are in maybe face-to-face conversations, but I'd really like to understand from you what customers are asking for in terms of moving design solutions, engineering solutions to the cloud. I have to imagine that there's a range of demand. Our research shows that some companies really favor the cloud and they are looking in a cloud-first mode. On the other hand, we've got companies that say that they have policies that restrict their use of the cloud, whether it's an internal policy, a governmental regulation, or a customer policy. But most companies are really looking for functionality first and then looking for deployment after the fact and just trying to find the solution that really helps them get their job done. What are you hearing your customers ask for when it comes to engineering solutions on the cloud?
Paul: Well, Jim, it's interesting because you say, you see the multiple different camps of opinions when we look at the cloud, but I think the people that are really looking at cloud are really looking at what can this do to add value to my business? There are always going to be, as you said, there are some people that are, for whatever reason, not going to adopt cloud. They're military, they have systems that have air gaps, so using external cloud technologies may not help them, but in many cases, they're looking at what they can do internally into their network to be able to do that. But then there are other customers who are looking and saying, "Okay, well, how do I leverage the cloud to give me benefits and benefits in my business?" What most designers are not prepared to do is give up functionality. So if you say, "Well, you have to use a skinnied-down system," most engineers, both design engineers, simulation engineers, throw up their hands in horror because they get... You guarantee that they would never be prepared to give up any piece of functionality if it means... No matter what the offer is.
Because everyone gets into it, they know and love the functionality, so what we're seeing in many companies is they're looking for an extension, they're looking for ways that cloud type technologies can help their businesses, whether it's better collaboration, whether it's easier access, whether it's... There are niche applications, very, very specific applications that are targeted at a particular function where the person is not using the system all the time, but wants to leverage the tool. So that's really what we're seeing, is this mixture of desktop applications, but with the added value delivered by cloud as being a more, in many ways a more popular solution than just a wholesale, "I'm going to switch off all my desktops and move everyone to the cloud" as a norm. I think you're seeing this transition period.
Jim: Yeah, I think that lines up very nicely, Paul, with what we're seeing, is that companies are telling us that they're not transitioning to the cloud for cloud's sake. They value the benefits of the cloud, but in the end, they need to meet their targets. They've got innovation, quality, product performance, and time targets that they need to meet. When you think about this transition to the cloud, how does Siemens see customers making that transition? How do you envision that, and how are you supporting them through that process?
Paul: Well, I think there is a few important points about the way that we're trying to support them through this process, and that's giving them the access that they want. Importantly, the first thing is, we're not trying to force them down a particular route, so we're happy for them to go their own pace, their own way, and decide how they want to deploy this, and in many cases, it might be that they end up in this hybrid environment, this mixed environment, having software on desktop and software in cloud connecting to get the two together. Importantly, I think the underpinning of that, though, we do a lot of work to make sure that the architectures of our products work across the different environments, whether it's cloud or desktop, so we don't want to find... When we introduce things like Teamcenter X, it works across the entire platform of products. With NX, making sure that anything we do when we're streaming, when we're using cloud type technologies or desktop technologies, that the data is compatible because at the end of the day, the data is what is the customer's intellectual property, and that's where their value is.
We don't want to put them in a situation where they've got to choose, firstly, reductions of functionality, but also things like data translation, moving data around, that is not the type of way we look at these things. We say we want to make it as easy and simple as possible to access the cloud, leverage the benefits of the cloud, but without losing your intellectual property in any way, shape, or form.
Jim: Paul, thanks, and as you're looking forward into the future, do you think that there are certain areas that your customers will look for sooner rather than others? I know I'm asking you a little bit to leverage some of your knowledge of your customers, but also take out the Paul Brown crystal ball and try and understand your view based on your experience. What types of solutions do you see customers adopting first, or is it really just something that's individualized and specialized to each individual customer? Are there trends?
Paul: I think for a lot of the customers that we're talking to, they're looking at ways that they can expand out their usage by leveraging the cloud. They're not just looking at, okay, so I've got CAD on the desktop, got our design on the desktop or engineering on the desktop, and I just want to move it to the cloud because the cloud is cool or the cloud is as far as they're looking at, okay, how can it help expand out my usage? For example, one of the key topics in the mold business for our mold customers is being able to do accurate quoting and being able to estimate such that when they put their quotes out, that they have a high degree of confidence. But that group doing that are not traditional CAD users, but imagine now if by tying together our design tools for the molding, things like our costing tools that are a part of our Teamcenter product, and delivering that over cloud on-demand when people want to use it to do their bids, to do their processes.
That's leveraging the cloud to get a value, and it is expanding out the value of the tools and the digital twin to those customers, versus just a straight swap. And I think that that's what we're seeing, and I'm seeing. They are certain applications, extra applications, more extensions of what they do versus just saying, "Okay, I'm doing core modeling or I'm doing core drafting or core CIM." It's really, how can I get more people involved in the process, because I have got this flexibility and scalability of the cloud.
Jim: Yeah, so not just swapping out what they could do before for a different architecture stack, but really trying to do more and extend value. I think that's a great message. Paul, thank you so much for joining me. I look forward to the days where we can have these conversations in person again, perhaps in a cocktail setting, at a user conference. But for now, I really appreciate the ability to have these conversations virtually. Thank you a lot.
Paul: Absolutely. Thanks, Jim.
[post_title] => Sharing Ideas on Cloud Adoption with Siemens (podcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-adoption-podcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:32:26
[post_modified_gmt] => 2022-12-02 20:32:26
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9885
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[3] => WP_Post Object
(
[ID] => 9918
[post_author] => 2574
[post_date] => 2020-11-02 10:35:18
[post_date_gmt] => 2020-11-02 15:35:18
[post_content] =>
You can also see related video interviews including: Engineering in the Cloud Conversation and Designing on the Cloud Discussion with Paul Brown, Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell and Siemens Digital Transformation Progress with Brenda Discher.
Transcript:
Jim: Hi, I'm Jim Brown, President of Tech-Clarity, where we make the business value of technology clear. Today, I'm joined again by Paul Brown, Senior Marketing Director at Siemens Digital Industries Software. Paul's been very generous with his time. We've had a chance on this podcast to discuss design solutions moving to the cloud, things like CAD, but also a broader conversation about engineering software moving to the cloud, including things like visualization, rendering, simulation, and a little bit broader conversation about digital transformation. Now I want to really ask Paul, how do engineers adopt these solutions? How do engineers adopt these solutions? Let's get some clarity.
Paul, welcome, always a pleasure, I appreciate you coming back onto the podcast.
Paul: It's great to be here, Jim.
Jim: Paul, I know that you get a chance to speak with customers, and I know we're probably in more virtual conversations today than we are in maybe face-to-face conversations, but I'd really like to understand from you what customers are asking for in terms of moving design solutions, engineering solutions to the cloud. I have to imagine that there's a range of demand. Our research shows that some companies really favor the cloud and they are looking in a cloud-first mode. On the other hand, we've got companies that say that they have policies that restrict their use of the cloud, whether it's an internal policy, a governmental regulation, or a customer policy. But most companies are really looking for functionality first and then looking for deployment after the fact and just trying to find the solution that really helps them get their job done. What are you hearing your customers ask for when it comes to engineering solutions on the cloud?
Paul: Well, Jim, it's interesting because you say, you see the multiple different camps of opinions when we look at the cloud, but I think the people that are really looking at cloud are really looking at what can this do to add value to my business? There are always going to be, as you said, there are some people that are, for whatever reason, not going to adopt cloud. They're military, they have systems that have air gaps, so using external cloud technologies may not help them, but in many cases, they're looking at what they can do internally into their network to be able to do that. But then there are other customers who are looking and saying, "Okay, well, how do I leverage the cloud to give me benefits and benefits in my business?" What most designers are not prepared to do is give up functionality. So if you say, "Well, you have to use a skinnied-down system," most engineers, both design engineers, simulation engineers, throw up their hands in horror because they get... You guarantee that they would never be prepared to give up any piece of functionality if it means... No matter what the offer is.
Because everyone gets into it, they know and love the functionality, so what we're seeing in many companies is they're looking for an extension, they're looking for ways that cloud type technologies can help their businesses, whether it's better collaboration, whether it's easier access, whether it's... There are niche applications, very, very specific applications that are targeted at a particular function where the person is not using the system all the time, but wants to leverage the tool. So that's really what we're seeing, is this mixture of desktop applications, but with the added value delivered by cloud as being a more, in many ways a more popular solution than just a wholesale, "I'm going to switch off all my desktops and move everyone to the cloud" as a norm. I think you're seeing this transition period.
Jim: Yeah, I think that lines up very nicely, Paul, with what we're seeing, is that companies are telling us that they're not transitioning to the cloud for cloud's sake. They value the benefits of the cloud, but in the end, they need to meet their targets. They've got innovation, quality, product performance, and time targets that they need to meet. When you think about this transition to the cloud, how does Siemens see customers making that transition? How do you envision that, and how are you supporting them through that process?
Paul: Well, I think there is a few important points about the way that we're trying to support them through this process, and that's giving them the access that they want. Importantly, the first thing is, we're not trying to force them down a particular route, so we're happy for them to go their own pace, their own way, and decide how they want to deploy this, and in many cases, it might be that they end up in this hybrid environment, this mixed environment, having software on desktop and software in cloud connecting to get the two together. Importantly, I think the underpinning of that, though, we do a lot of work to make sure that the architectures of our products work across the different environments, whether it's cloud or desktop, so we don't want to find... When we introduce things like Teamcenter X, it works across the entire platform of products. With NX, making sure that anything we do when we're streaming, when we're using cloud type technologies or desktop technologies, that the data is compatible because at the end of the day, the data is what is the customer's intellectual property, and that's where their value is.
We don't want to put them in a situation where they've got to choose, firstly, reductions of functionality, but also things like data translation, moving data around, that is not the type of way we look at these things. We say we want to make it as easy and simple as possible to access the cloud, leverage the benefits of the cloud, but without losing your intellectual property in any way, shape, or form.
Jim: Paul, thanks, and as you're looking forward into the future, do you think that there are certain areas that your customers will look for sooner rather than others? I know I'm asking you a little bit to leverage some of your knowledge of your customers, but also take out the Paul Brown crystal ball and try and understand your view based on your experience. What types of solutions do you see customers adopting first, or is it really just something that's individualized and specialized to each individual customer? Are there trends?
Paul: I think for a lot of the customers that we're talking to, they're looking at ways that they can expand out their usage by leveraging the cloud. They're not just looking at, okay, so I've got CAD on the desktop, got our design on the desktop or engineering on the desktop, and I just want to move it to the cloud because the cloud is cool or the cloud is as far as they're looking at, okay, how can it help expand out my usage? For example, one of the key topics in the mold business for our mold customers is being able to do accurate quoting and being able to estimate such that when they put their quotes out, that they have a high degree of confidence. But that group doing that are not traditional CAD users, but imagine now if by tying together our design tools for the molding, things like our costing tools that are a part of our Teamcenter product, and delivering that over cloud on-demand when people want to use it to do their bids, to do their processes.
That's leveraging the cloud to get a value, and it is expanding out the value of the tools and the digital twin to those customers, versus just a straight swap. And I think that that's what we're seeing, and I'm seeing. They are certain applications, extra applications, more extensions of what they do versus just saying, "Okay, I'm doing core modeling or I'm doing core drafting or core CIM." It's really, how can I get more people involved in the process, because I have got this flexibility and scalability of the cloud.
Jim: Yeah, so not just swapping out what they could do before for a different architecture stack, but really trying to do more and extend value. I think that's a great message. Paul, thank you so much for joining me. I look forward to the days where we can have these conversations in person again, perhaps in a cocktail setting, at a user conference. But for now, I really appreciate the ability to have these conversations virtually. Thank you a lot.
Paul: Absolutely. Thanks, Jim.
[post_title] => Sharing Ideas on Cloud Adoption with Siemens (podcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-adoption-podcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:32:26
[post_modified_gmt] => 2022-12-02 20:32:26
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9885
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[3] => WP_Post Object
(
[ID] => 9918
[post_author] => 2574
[post_date] => 2020-11-02 10:35:18
[post_date_gmt] => 2020-11-02 15:35:18
[post_content] =>  What happens when automation and plant IT teams get past their differences and work together effectively?
A chance for the company to outperform the competition and make progress on Industry 4.0 projects, based on more coherent manufacturing data management.
In our recent research, Top Performers were more likely to have tightly connected IT and automation or operations technology (OT) organizations. This article provides highlights of the recent research, The Manufacturing Data Challenge: Lessons from Top Performers.
The article touches on investments, integration, and where Top Performers really outshine others in people, process, and technology.
Please visit Automation World to read the article.
Please visit our sponsor, Critical Manufacturing, for the full research (registration required).
[post_title] => The Challenge of Manufacturing Data Management (article)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => manufacturing-data-management-article
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:56
[post_modified_gmt] => 2022-11-15 03:25:56
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9918
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[4] => WP_Post Object
(
[ID] => 9830
[post_author] => 2
[post_date] => 2020-10-28 08:00:52
[post_date_gmt] => 2020-10-28 12:00:52
[post_content] =>
What happens when automation and plant IT teams get past their differences and work together effectively?
A chance for the company to outperform the competition and make progress on Industry 4.0 projects, based on more coherent manufacturing data management.
In our recent research, Top Performers were more likely to have tightly connected IT and automation or operations technology (OT) organizations. This article provides highlights of the recent research, The Manufacturing Data Challenge: Lessons from Top Performers.
The article touches on investments, integration, and where Top Performers really outshine others in people, process, and technology.
Please visit Automation World to read the article.
Please visit our sponsor, Critical Manufacturing, for the full research (registration required).
[post_title] => The Challenge of Manufacturing Data Management (article)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => manufacturing-data-management-article
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:56
[post_modified_gmt] => 2022-11-15 03:25:56
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9918
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[4] => WP_Post Object
(
[ID] => 9830
[post_author] => 2
[post_date] => 2020-10-28 08:00:52
[post_date_gmt] => 2020-10-28 12:00:52
[post_content] =>  How is the availability of cloud applications for engineering changing the way people work? Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives.
[audio mp3="https://tech-clarity.com/wp-content/uploads/2020/10/Engineering-in-the-Cloud-podcast.mp3"][/audio]
How is the availability of cloud applications for engineering changing the way people work? Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives.
[audio mp3="https://tech-clarity.com/wp-content/uploads/2020/10/Engineering-in-the-Cloud-podcast.mp3"][/audio]
 You can also see related video interviews including: Designing on the Cloud Discussion with Paul Brown, Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell and Siemens Digital Transformation Progress with Brenda Discher.
Transcript:
Jim Brown: Hi, I'm Jim Brown, President of Tech-Clarity, where we make the business value of technology clear. Today, I'm joined again by Paul Brown, Senior Marketing Director at Siemens Digital Industries Software. We had a great chance, earlier, to talk about the increased adoption of the cloud in product innovation and engineering. We talked about the changes in the areas of design and supporting digital transformation, and how people are changing the way they work. But how does that apply to the broader topic of engineering in the cloud? Let's get some clarity.
Paul, welcome.
Paul Brown: Hi, Jim. Good to talk to you again.
JIM: Always a pleasure. Paul, last time, we talked about the fact that cloud has values in lots of different areas, in terms of implementation benefits, adoption benefits, operational benefits, and business benefits. There are so many ways that the cloud is changing the world, but we also have seen special areas that it provides even more unique value, in areas of engineering. And some of that has to do with infinite computing versus companies having to maintain banks and banks of servers, and having to continually maintain and replace expensive hardware, for things like generative design that benefit from a tremendous amount of horsepower to drive simulation and iteration, also things like collaboration. In your experience, what types of engineering and engineering applications are best suited for the cloud?
PAUL: Well, it's a good question, Jim, because, as you rightly say, there's a kind of very traditional way of looking at the engineering processes of simulation, and bringing in multi-physics, multiple types of simulation into the process. And for a lot of companies, they're not doing this all the time, they have cyclic needs, depending on where they are in a project, it brings the need for increased power, increased compute power, but also increased flexibility of being able to use that. So, whether it's on, as you mentioned, the traditional way of looking at engineering as simulation, and the computer-aided engineering approaches of structural, thermal, computational fluid dynamics, but we're also seeing it with companies looking at other types of advanced simulations.
Whether it's factory layout, for example. "Can I do a process flow inside of a brand-new build factory to actually analyze the flow of material and optimize that flow of materials?" So going outside of the traditional engineering and into more production engineering type of areas, bringing those types of analysis together. But when you think about it, those are not analysis you're going to be doing every day, they're analysis you'll do a number of times in the project. But the project could span a period of months, it could be six months, it could be a year. And you don't want to have expensive hardware, high-end processing power around doing nothing, so being able to access that level of flexibility and access the software in that way, in a very flexible way, is how you can leverage the cloud. You can actually use the cloud solutions to be able to get access to the software that you need when you need it, and with the power that you need it. It's kind of opening up the power of the entire suite, and it's actually allowing us to expand out the use of the digital twin, with more validation and more optimization and more simulations.
JIM: Great. Thanks Paul. I think those are great examples. Let's drill down a little bit more on scalability from a hardware and a horsepower perspective. Our research and some recent interviews that we've done really show that companies value that power of cloud for scalability. And the ability to access the power that they need for solutions that require a lot of heavy lifting, and those that, as you said earlier, may be something that happens intermittently. You mentioned some simulations of different kinds, I think that's a great example. Are there other kinds of solutions that require a lot of compute power and how does the cloud really help with that?
PAUL: There are. I think, obviously, the traditional one, as I mentioned, everyone looks at the traditional engineering simulations, the structural, the thermal. But there are other areas to leverage the digital twin that we are seeing more people would use. Even things like visualization, it's kind of a form of simulation because often you're looking at a visualization and animation, and to get the really high-end rendering takes a lot of processing power, so you're left with either the need to have expensive graphics engines on-site or you can actually start leveraging the cloud and having your cloud served GPU type access.
So instead of just relying on traditional CPU calculations, actually putting things through graphics processors and having banks of graphic processors that allow you to then farm visualization and dramatically reduce the amount of time it's taking to do things like rendering or animations. And being able to do that on a design model, once again, makes the digital twin richer, because you can start using it, you can start doing things like animating it and showing it's behavior, really bringing that power in, but without having to invest in, say, massive amounts of compute power that do this.
We dealt with one customer and they would spend literally days rendering high quality imagery of their products. Their products were... I wouldn't say... Well, they were complex, but if you looked, they weren't particularly massive products, maybe 10,000 components making up a product. And they wanted to visualize this, because it's in a marketplace which is driven by consumer buying power, so it relies on people who want that desire to own. And being able to generate these high-end renderings was taking them days and being able to shorten that time by farming this out and then maybe overnight on a GPU farm, being able to then get the rendering and then they work with it, navigate it, rotate it, look at it and get a much better, much higher confidence about the quality of the product that they've got. And I think that that's a sort of analysis, that's a sort of use of that scalability without it necessarily being just purely around compute power, it can be graphics power too.
JIM: Thanks, Paul, I appreciate that. And one of the things that we see and we hear from companies as we speak to them is that, like hardware and the need for scalability there, companies have the same need for their software. They've got lumpy demand for their software where they have a variety of different apps, and some of the apps have gotten very specialized. And that maybe they need to access different applications at different times where they may use something intermittently, for example, something like crash analysis that they might use towards the end of a program.
Or somebody might be doing some early analysis on systems engineering very early in the process. And that some of those more specialized tools may be things that would sit on the shelf and not be used, where with the cloud, oftentimes the access to those things is a little bit more flexible as well. And we also see companies that have scalability from programs, for example, they may be running a large program and then roll off of one program and then there may be a gap in time before the next program. So from both a software perspective and a user and business perspective, there's a lot of value in the cloud in terms of that kind of scalability or flexibility as well. Is that something that you're seeing as you're working with your customers?
PAUL: Yeah. Absolutely. I think we're seeing this need, people are now in a model where, as you mentioned, somebody might start a project out and they want to do a lot of analysis using a 1D solution for systems engineering to plan out their systems in the process. And once just the analysis is there and it's driving the design phase, you don't want to have to have that software. Many companies are looking to say, "Okay. Well, I don't need that software all the time." You talk about the complex analysis, and you look at some of these things, like cooling analysis. Well, maybe I only use that one month a year or something in terms of total time. Companies are looking to say, "Okay. Well, how can I get access to the software that I need when I need it?"
And be flexible, be able to say, "Okay. Well, today I've got this problem and it's a cooling problem. Tomorrow I've got a problem and it's a dynamics problem, and I'm trying to do response dynamics, and not be tied in to having to go off and get lots of niche software which I then have to install and manage and maintain, because that's the other thing that people always need to remember is, buying the software is one element. Put your software purchase is great, you then install it, you have to maintain it, you have to make sure that you're upgrading it. There's a constant thing. Well, and companies are now looking for people like ourselves to deliver those solutions where we are maintaining them, where we are offering them the most current versions, where we are making sure that it's all up and running and it's accessible as they need it and when they need it. And for the time that they need it, rather than saying, this is a, "No. You've purchased this and I don't mind how long you use it, but you have to buy it."
So it gives us a much more flexible approach to doing business, and I think that's a key element, is we want to be, as a partner, as flexible as possible for companies as they move into this environment. We're not trying to push people into a particular solution because it suits us. We want people to go at their pace, their own way. We want to make it accessible, usable for our customers, but at the end of the day, our customers have to be in control of the way that they want to run their business.
JIM: Yeah, thank you, Paul. And as you were talking, one of the things that also came to mind for me is companies, oftentimes, as they install software and have to maintain it, they get into that upgrade lag cycle. And we often hear quite a bit of companies talking about the fact that using the cloud when there's new capabilities that become available, they have access to them much faster as opposed to having to wait for an upgrade cycle or going to the user conference, back when we went to user conferences. And hearing about all of the great new functionality but realizing it was going take a year or two before they get it, so just another way we hear people talk about the value of the cloud.
PAUL: No. Absolutely, I think that that whole software lag, I think is something that people often underestimate, because if you're not careful, you get yourself into the business comfort zone, and it's kind of working for us, but the newer and the faster and the improved... Because vendors like ourselves are constantly improving our products, we're constantly improving the security, the performance of our products, and every time somebody puts that lag in the process, the people that get hurt are the end users. So they're the ones that are not seeing the latest, greatest, fastest. So yes, the cloud helps people overcome some of that barrier too.
JIM: And translates to getting programs done better and faster as well. Paul, thank you so much for extending our previous conversation about design in the cloud and really covering a broader aspect of engineering. Always a pleasure, I always learn something when I talk to you, thank you so much.
PAUL: Thanks, Jim, it was great to talk to you.
[post_title] => Engineering in the Cloud Conversation with Siemens (podcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => engineering-in-the-cloud-podcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:32:51
[post_modified_gmt] => 2022-12-02 20:32:51
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9830
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[5] => WP_Post Object
(
[ID] => 9858
[post_author] => 2
[post_date] => 2020-10-14 20:50:40
[post_date_gmt] => 2020-10-15 00:50:40
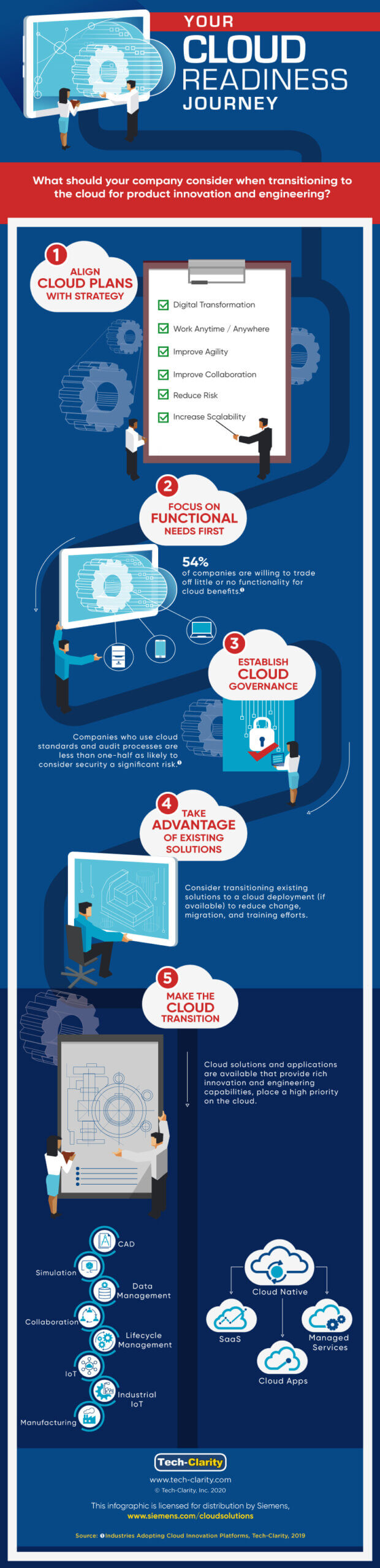
[post_content] => What should your company consider when transitioning to the cloud for innovation and engineering? Our new infographic identifies key considerations ranging from strategy through adoption.
See the infographics for some important factors related to strategy, functionality, governance, existing solutions, and your transition.
You can also learn more about cloud solutions from Siemens Digital Industries Software, our sponsor or view our video series sharing insights from our research and interviews including: Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell, and Siemens Digital Transformation Progress with Brenda Discher.
You can also see related video interviews including: Designing on the Cloud Discussion with Paul Brown, Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell and Siemens Digital Transformation Progress with Brenda Discher.
Transcript:
Jim Brown: Hi, I'm Jim Brown, President of Tech-Clarity, where we make the business value of technology clear. Today, I'm joined again by Paul Brown, Senior Marketing Director at Siemens Digital Industries Software. We had a great chance, earlier, to talk about the increased adoption of the cloud in product innovation and engineering. We talked about the changes in the areas of design and supporting digital transformation, and how people are changing the way they work. But how does that apply to the broader topic of engineering in the cloud? Let's get some clarity.
Paul, welcome.
Paul Brown: Hi, Jim. Good to talk to you again.
JIM: Always a pleasure. Paul, last time, we talked about the fact that cloud has values in lots of different areas, in terms of implementation benefits, adoption benefits, operational benefits, and business benefits. There are so many ways that the cloud is changing the world, but we also have seen special areas that it provides even more unique value, in areas of engineering. And some of that has to do with infinite computing versus companies having to maintain banks and banks of servers, and having to continually maintain and replace expensive hardware, for things like generative design that benefit from a tremendous amount of horsepower to drive simulation and iteration, also things like collaboration. In your experience, what types of engineering and engineering applications are best suited for the cloud?
PAUL: Well, it's a good question, Jim, because, as you rightly say, there's a kind of very traditional way of looking at the engineering processes of simulation, and bringing in multi-physics, multiple types of simulation into the process. And for a lot of companies, they're not doing this all the time, they have cyclic needs, depending on where they are in a project, it brings the need for increased power, increased compute power, but also increased flexibility of being able to use that. So, whether it's on, as you mentioned, the traditional way of looking at engineering as simulation, and the computer-aided engineering approaches of structural, thermal, computational fluid dynamics, but we're also seeing it with companies looking at other types of advanced simulations.
Whether it's factory layout, for example. "Can I do a process flow inside of a brand-new build factory to actually analyze the flow of material and optimize that flow of materials?" So going outside of the traditional engineering and into more production engineering type of areas, bringing those types of analysis together. But when you think about it, those are not analysis you're going to be doing every day, they're analysis you'll do a number of times in the project. But the project could span a period of months, it could be six months, it could be a year. And you don't want to have expensive hardware, high-end processing power around doing nothing, so being able to access that level of flexibility and access the software in that way, in a very flexible way, is how you can leverage the cloud. You can actually use the cloud solutions to be able to get access to the software that you need when you need it, and with the power that you need it. It's kind of opening up the power of the entire suite, and it's actually allowing us to expand out the use of the digital twin, with more validation and more optimization and more simulations.
JIM: Great. Thanks Paul. I think those are great examples. Let's drill down a little bit more on scalability from a hardware and a horsepower perspective. Our research and some recent interviews that we've done really show that companies value that power of cloud for scalability. And the ability to access the power that they need for solutions that require a lot of heavy lifting, and those that, as you said earlier, may be something that happens intermittently. You mentioned some simulations of different kinds, I think that's a great example. Are there other kinds of solutions that require a lot of compute power and how does the cloud really help with that?
PAUL: There are. I think, obviously, the traditional one, as I mentioned, everyone looks at the traditional engineering simulations, the structural, the thermal. But there are other areas to leverage the digital twin that we are seeing more people would use. Even things like visualization, it's kind of a form of simulation because often you're looking at a visualization and animation, and to get the really high-end rendering takes a lot of processing power, so you're left with either the need to have expensive graphics engines on-site or you can actually start leveraging the cloud and having your cloud served GPU type access.
So instead of just relying on traditional CPU calculations, actually putting things through graphics processors and having banks of graphic processors that allow you to then farm visualization and dramatically reduce the amount of time it's taking to do things like rendering or animations. And being able to do that on a design model, once again, makes the digital twin richer, because you can start using it, you can start doing things like animating it and showing it's behavior, really bringing that power in, but without having to invest in, say, massive amounts of compute power that do this.
We dealt with one customer and they would spend literally days rendering high quality imagery of their products. Their products were... I wouldn't say... Well, they were complex, but if you looked, they weren't particularly massive products, maybe 10,000 components making up a product. And they wanted to visualize this, because it's in a marketplace which is driven by consumer buying power, so it relies on people who want that desire to own. And being able to generate these high-end renderings was taking them days and being able to shorten that time by farming this out and then maybe overnight on a GPU farm, being able to then get the rendering and then they work with it, navigate it, rotate it, look at it and get a much better, much higher confidence about the quality of the product that they've got. And I think that that's a sort of analysis, that's a sort of use of that scalability without it necessarily being just purely around compute power, it can be graphics power too.
JIM: Thanks, Paul, I appreciate that. And one of the things that we see and we hear from companies as we speak to them is that, like hardware and the need for scalability there, companies have the same need for their software. They've got lumpy demand for their software where they have a variety of different apps, and some of the apps have gotten very specialized. And that maybe they need to access different applications at different times where they may use something intermittently, for example, something like crash analysis that they might use towards the end of a program.
Or somebody might be doing some early analysis on systems engineering very early in the process. And that some of those more specialized tools may be things that would sit on the shelf and not be used, where with the cloud, oftentimes the access to those things is a little bit more flexible as well. And we also see companies that have scalability from programs, for example, they may be running a large program and then roll off of one program and then there may be a gap in time before the next program. So from both a software perspective and a user and business perspective, there's a lot of value in the cloud in terms of that kind of scalability or flexibility as well. Is that something that you're seeing as you're working with your customers?
PAUL: Yeah. Absolutely. I think we're seeing this need, people are now in a model where, as you mentioned, somebody might start a project out and they want to do a lot of analysis using a 1D solution for systems engineering to plan out their systems in the process. And once just the analysis is there and it's driving the design phase, you don't want to have to have that software. Many companies are looking to say, "Okay. Well, I don't need that software all the time." You talk about the complex analysis, and you look at some of these things, like cooling analysis. Well, maybe I only use that one month a year or something in terms of total time. Companies are looking to say, "Okay. Well, how can I get access to the software that I need when I need it?"
And be flexible, be able to say, "Okay. Well, today I've got this problem and it's a cooling problem. Tomorrow I've got a problem and it's a dynamics problem, and I'm trying to do response dynamics, and not be tied in to having to go off and get lots of niche software which I then have to install and manage and maintain, because that's the other thing that people always need to remember is, buying the software is one element. Put your software purchase is great, you then install it, you have to maintain it, you have to make sure that you're upgrading it. There's a constant thing. Well, and companies are now looking for people like ourselves to deliver those solutions where we are maintaining them, where we are offering them the most current versions, where we are making sure that it's all up and running and it's accessible as they need it and when they need it. And for the time that they need it, rather than saying, this is a, "No. You've purchased this and I don't mind how long you use it, but you have to buy it."
So it gives us a much more flexible approach to doing business, and I think that's a key element, is we want to be, as a partner, as flexible as possible for companies as they move into this environment. We're not trying to push people into a particular solution because it suits us. We want people to go at their pace, their own way. We want to make it accessible, usable for our customers, but at the end of the day, our customers have to be in control of the way that they want to run their business.
JIM: Yeah, thank you, Paul. And as you were talking, one of the things that also came to mind for me is companies, oftentimes, as they install software and have to maintain it, they get into that upgrade lag cycle. And we often hear quite a bit of companies talking about the fact that using the cloud when there's new capabilities that become available, they have access to them much faster as opposed to having to wait for an upgrade cycle or going to the user conference, back when we went to user conferences. And hearing about all of the great new functionality but realizing it was going take a year or two before they get it, so just another way we hear people talk about the value of the cloud.
PAUL: No. Absolutely, I think that that whole software lag, I think is something that people often underestimate, because if you're not careful, you get yourself into the business comfort zone, and it's kind of working for us, but the newer and the faster and the improved... Because vendors like ourselves are constantly improving our products, we're constantly improving the security, the performance of our products, and every time somebody puts that lag in the process, the people that get hurt are the end users. So they're the ones that are not seeing the latest, greatest, fastest. So yes, the cloud helps people overcome some of that barrier too.
JIM: And translates to getting programs done better and faster as well. Paul, thank you so much for extending our previous conversation about design in the cloud and really covering a broader aspect of engineering. Always a pleasure, I always learn something when I talk to you, thank you so much.
PAUL: Thanks, Jim, it was great to talk to you.
[post_title] => Engineering in the Cloud Conversation with Siemens (podcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => engineering-in-the-cloud-podcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:32:51
[post_modified_gmt] => 2022-12-02 20:32:51
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9830
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[5] => WP_Post Object
(
[ID] => 9858
[post_author] => 2
[post_date] => 2020-10-14 20:50:40
[post_date_gmt] => 2020-10-15 00:50:40
[post_content] => What should your company consider when transitioning to the cloud for innovation and engineering? Our new infographic identifies key considerations ranging from strategy through adoption.
See the infographics for some important factors related to strategy, functionality, governance, existing solutions, and your transition.
You can also learn more about cloud solutions from Siemens Digital Industries Software, our sponsor or view our video series sharing insights from our research and interviews including: Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell, and Siemens Digital Transformation Progress with Brenda Discher.
 [post_title] => Cloud Readiness (infographic)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-readiness-infographic
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:09
[post_modified_gmt] => 2022-11-15 03:26:09
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9858
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[6] => WP_Post Object
(
[ID] => 9818
[post_author] => 2574
[post_date] => 2020-10-08 12:50:19
[post_date_gmt] => 2020-10-08 16:50:19
[post_content] =>
[post_title] => Cloud Readiness (infographic)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-readiness-infographic
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:26:09
[post_modified_gmt] => 2022-11-15 03:26:09
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9858
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[6] => WP_Post Object
(
[ID] => 9818
[post_author] => 2574
[post_date] => 2020-10-08 12:50:19
[post_date_gmt] => 2020-10-08 16:50:19
[post_content] =>  What do manufacturers need to do to succeed and get results from their Industry 4.0 efforts?
In this webcast, Julie Fraser revealed a few significant findings of research of over 300 companies on the topic of manufacturing data management. It’s a multi-faceted challenge in which people, processes, and technology all matter.
This webcast explains the new Tech-Clarity research report’s findings, The Manufacturing Data Challenge: Lessons from Top Performers. Attend to learn what Top Performers are doing differently than others to improve dramatically on both operational and business key performance indicators (KPIs).
Register now to hear about this ground-breaking research, sponsored by Critical Manufacturing (registration required).
[post_title] => Meeting the Manufacturing Data Management Challenge (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => manufacturing-data-management-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:27:02
[post_modified_gmt] => 2022-11-15 03:27:02
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9818
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[7] => WP_Post Object
(
[ID] => 9673
[post_author] => 2574
[post_date] => 2020-10-02 09:39:54
[post_date_gmt] => 2020-10-02 13:39:54
[post_content] =>
What do manufacturers need to do to succeed and get results from their Industry 4.0 efforts?
In this webcast, Julie Fraser revealed a few significant findings of research of over 300 companies on the topic of manufacturing data management. It’s a multi-faceted challenge in which people, processes, and technology all matter.
This webcast explains the new Tech-Clarity research report’s findings, The Manufacturing Data Challenge: Lessons from Top Performers. Attend to learn what Top Performers are doing differently than others to improve dramatically on both operational and business key performance indicators (KPIs).
Register now to hear about this ground-breaking research, sponsored by Critical Manufacturing (registration required).
[post_title] => Meeting the Manufacturing Data Management Challenge (webcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => manufacturing-data-management-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:27:02
[post_modified_gmt] => 2022-11-15 03:27:02
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9818
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[7] => WP_Post Object
(
[ID] => 9673
[post_author] => 2574
[post_date] => 2020-10-02 09:39:54
[post_date_gmt] => 2020-10-02 13:39:54
[post_content] =>  How do companies make progress toward Industry 4.0? Based on our research, those who invest in people, processes, and technology for manufacturing data management have made more strides in Industry 4.0. Manufacturing Data Management: Lessons from Top Performers explores the many challenges of bringing together all the data production facilities need.
Please enjoy the summary* below. For the full research, please visit our sponsor Critical Manufacturing (registration required).
How do companies make progress toward Industry 4.0? Based on our research, those who invest in people, processes, and technology for manufacturing data management have made more strides in Industry 4.0. Manufacturing Data Management: Lessons from Top Performers explores the many challenges of bringing together all the data production facilities need.
Please enjoy the summary* below. For the full research, please visit our sponsor Critical Manufacturing (registration required).
Table of Contents
- Vision for Business Breakthroughs
- Keys to Industry 4.0 Success
- Manufacturing Data Management Activity
- Vertical Integration
- Challenges in the Current State
- Defining Plant Operations Success
- Top Performers Make Progress
- Organizational Issues
- Staffing for Manufacturing Data Programs
- Viewpoints on Skills
- Manufacturing Data Management Initiatives
- What Does It Take?
- Technology: Feeding Success?
- Manufacturing Data Approaches
- Business Impact
- Conclusions
- Recommendations
- About the Research
- Acknowledgments
Executive Overview
Prioritizing Manufacturing Data Management What have manufacturers been struggling with for years that Industry 4.0 insists they solve now? Manufacturing data management. Combining data from information technology (IT) and operations technology (OT) is essential to analysis and insights. Thus, coherent and consistent plant data is a foundation for achieving the agility and business value companies want. Yet it is anything but straightforward. And it’s not the only thing companies need to meet the challenge of manufacturing data. Research Reveals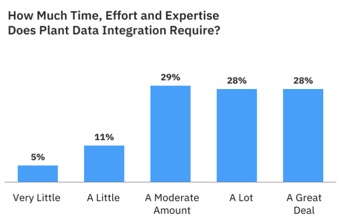 This research aims to learn about current challenges, strategies, and programs for manufacturing data management. Over 300 responded, from manufacturers in various industry segments and of all sizes, working with data from plants in every corner of the world. We uncovered a significant and nearly universal outstanding need: better ways to integrate IT and OT data. We also discovered that there are paths that appear to lead to greater success for those who travel them most aggressively.
Top Performers have made mindset, organization, and staffing changes. They are also far more likely to be using commercial software solutions and adopting modern approaches to accelerate their ability to manage manufacturing data effectively. As a result, a larger percentage of these Top Performers have already made dramatic improvements in business performance. They are in a position to make more gains.
This research aims to learn about current challenges, strategies, and programs for manufacturing data management. Over 300 responded, from manufacturers in various industry segments and of all sizes, working with data from plants in every corner of the world. We uncovered a significant and nearly universal outstanding need: better ways to integrate IT and OT data. We also discovered that there are paths that appear to lead to greater success for those who travel them most aggressively.
Top Performers have made mindset, organization, and staffing changes. They are also far more likely to be using commercial software solutions and adopting modern approaches to accelerate their ability to manage manufacturing data effectively. As a result, a larger percentage of these Top Performers have already made dramatic improvements in business performance. They are in a position to make more gains.
Vision for Business Breakthroughs
The Status Quo is Risky Many companies are now pursuing digital transformation for a new and dramatically different future. With tremendous amounts of data at every level, but especially in production plants, manufacturers know they must do a better job using that data for business decisions. And manufacturing data poses special issues. Most Are on a Path to Industry 4.0 This vision of manufacturing transformation based on digital approaches is often called Industry 4.0. Most manufacturers in every industry and size range indicate that they have multiple Industry 4.0 projects underway. Traditional and Innovative Goals The goals are for agility and performance improvements on business-critical key performance indicators (KPIs). Some companies are also running a data-driven business to make more revenue from services or find other ways to add value.Recommendations for Manufacturing Data Management
The Path Forward Manufacturers must take action to compete with or stay a Top Performer. Formulating a clear strategy with business benefits for Industry 4.0 is a foundation. We recommend that manufacturers follow the lead of Top Performers:- Prioritize manufacturing data management and invest in both the staffing and the programs to succeed.
- Adopt proven commercial technologies such as MES, PLM, and APS where possible first. They will free up precious time and resources and provide a solid foundation for advanced approaches and analytics.
- Structure a program with projects that feed business needs, then proceed logically, in order of the process. For example, ensure data collection and enrichment are in place before investing in analytics projects.
- Create an environment where collaboration among disciplines feels natural, and the shared vision is more compelling than the inherent differences.
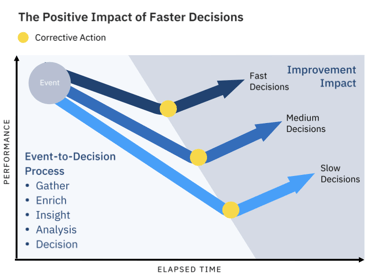 Even for Top Performers, challenges remain. These include data and systems integration, putting IT and OT data into a common context, agreeing on "sources of truth," and identifying data owners.
Note also that this is early days for coherent manufacturing data management. Yes, several times as many of the Top Performers have a program, staff members, capabilities, or dramatic gains on KPIs. Yet they are still only a minority.
There is more to do for every manufacturer. Faster decisions have always made a difference. However, doing things as we've done them may no longer suffice.
Seek New Approaches to Manufacturing Data Management
So, beyond doing what has been possible, we also encourage companies to seek new approaches. Solution providers are advancing their offerings. Some have integrated more functionality into plant systems. New offerings are becoming available to support manufacturing data management now and in the near future.
Be ready to explore new approaches. Validate their fit and ability to help meet your manufacturing data management challenges. Leverage both new and existing technologies and approaches to progress toward your Industry 4.0 vision.
Even for Top Performers, challenges remain. These include data and systems integration, putting IT and OT data into a common context, agreeing on "sources of truth," and identifying data owners.
Note also that this is early days for coherent manufacturing data management. Yes, several times as many of the Top Performers have a program, staff members, capabilities, or dramatic gains on KPIs. Yet they are still only a minority.
There is more to do for every manufacturer. Faster decisions have always made a difference. However, doing things as we've done them may no longer suffice.
Seek New Approaches to Manufacturing Data Management
So, beyond doing what has been possible, we also encourage companies to seek new approaches. Solution providers are advancing their offerings. Some have integrated more functionality into plant systems. New offerings are becoming available to support manufacturing data management now and in the near future.
Be ready to explore new approaches. Validate their fit and ability to help meet your manufacturing data management challenges. Leverage both new and existing technologies and approaches to progress toward your Industry 4.0 vision.
 How is the cloud playing a role in engineers' design processes?
Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives and examples.
[audio mp3="https://tech-clarity.com/wp-content/uploads/2020/09/Tech-Clarity-Cloud-Design-Podcast-2020-09-22.mp3"][/audio]
How is the cloud playing a role in engineers' design processes?
Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives and examples.
[audio mp3="https://tech-clarity.com/wp-content/uploads/2020/09/Tech-Clarity-Cloud-Design-Podcast-2020-09-22.mp3"][/audio]
 You can also see related video interviews including: Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell, and Siemens Digital Transformation Progress with Brenda Discher.
Transcript:
JIM: Hi, I'm Jim Brown, President of Tech-Clarity, where we make the business value of technology clear. Today, I'm joined by Paul Brown, a long-time industry leader and Senior Marketing Director at Siemens Digital Industries Software. We're going to talk to Paul about design engineering and how the cloud is really playing a big role there. Let's get some clarity.
JIM: Paul, thanks very much for joining me today.
PAUL: Yeah. Thanks, Jim.
JIM: So we've seen increased interest in the cloud in lots of different kinds of solutions, everything from probably email being run on the cloud for a long time, and ERP and others, but we're starting to see a lot of increased interest in design tools on the cloud and understanding how the process of design and engineering can evolve to leverage capabilities of the cloud. So there are obviously a lot of generic benefits of lower barriers to entry for a solution, whether it be cost or hardware, some lower risk and operational benefits around scalability of users and things along those needs, but we've also noticed that there are some really unique benefits for some engineering solutions, and maybe those come from enhanced collaboration or reach, but they may also come from increased scalability, access to "infinite computing power on demand." When you think about what's happening in design and how the cloud may be changing things, what do you think are the most important things for companies to know?
PAUL: Yeah, it's an interesting point, Jim. And I think we're definitely seeing a lot more interest in cloud-based solutions, but I will say, maybe this is a little bit controversial as a viewpoint, but at the moment, we're seeing a lot of interest, adoption is a bit more steady. I think that there's a lot more kind of people kind of, first of all, looking at what this cloud is going to do for them, so what that's really doing is it's actually getting a number of companies into this area where they've got a level of desktop application and they're starting to use cloud solutions combined together, so you end up with this kind of mixed environment. And really the important thing, and one thing I will say is, that's not a choice that we make or... That is a company choice that is down for the companies as they go forward.
PAUL: We can't, as vendors, force people to one or other approach just because it suits us. Okay, and we really have to look at letting customers decide and work at their own pace and their own way of getting forward in these types of technologies and use what's best for them. And that's always been one of the strategies here at Siemens, is that we've been looking at letting people work this way through and adjust, so the important thing is really is flexibility and being able to access the tools that they need to do their job as they need them and when they need them. I think when you start looking at why people, why the cloud is becoming more interesting, I think design really is, and product development is really a collaborative exercise. There are very few products that we see that are designed by a single person right the way through from start to finish, and so it relies on communicating with other people, sharing design work, and so one of the key elements that we're seeing is people are looking at leveraging the cloud to communicate between groups, to be able to make better design decisions, to share information.
PAUL: An example for you here, about two years ago, we introduced a product called NX Virtual Reality. So NX Virtual Reality first came out, it was great. You're inside your NX session, put on a headset, controllers, and you're immersed inside the product, you're one-to-one scale. We showed it to people. They loved it. I mean, people looked, "Yeah, well, it's... Yeah, it's great." But after a while, they kind of, their response starts coming as like, "Well, yeah, it's good, it gives me some benefits, but you know, yeah, it's okay, but... " There's always a but that comes on. Last year, what we did is we added in the multi-user version of virtual reality, so using multi-user, people can be dispersed around the globe, use a cloud-based solution and join in that session each with their own hardware, each with their own headsets, see each other, be doing work together, being able to measure things, move things, try things out, walk around their design in full-scale, and that's when people started coming back and saying, "Right, now I can do something different in my processes. I can bring people together."
PAUL: And that's because that we're leveraging the power of the cloud to bring together collaboration. I think there are other areas that we're seeing. We're seeing an increasing use of tools like generative engineering, where designers are leveraging the power of simulation and bringing simulation closer into the design process, into the creation process. So leveraging technologies like optimization, and particularly when you start getting into multi-disciplinary optimization, when you are not just looking at structural and stresses, but you're also combining structural and stresses with something like flow and computational fluid dynamics and you're trying to do these really complex type analyses, you need compute power.
PAUL: Now, your options are you have local machines with loads of power or you leverage the cloud, you leverage the compute power in the cloud. As you mentioned, that gives you that kind of near infinite compute power to help you do that and bring that simulation in to help you in that area. So it's giving you that more flexible infrastructure to be able to deliver the power when you need it to the people that need it. And I think those are the types of things that people are looking to actually say, "Okay, well, this is going to make a big difference in my product development process."
JIM: Paul, I really resonate with what you're saying of people looking for things that are not just, "I want to implement the latest technology or use the latest buzz word, but how am I going to change my workflow, how am I going to change my ability to do my job?" And it's interesting, we actually had some survey results along a similar line where we decided to ask companies for the benefits of the cloud, for all of the reduced cost and reduced risk and scalability, etcetera, etcetera, how much functionality are they willing to trade off for that? So basically, whether they're... Are they taking a cloud-first approach or a solution-first approach to their selections. And what we saw really overwhelmingly, the majority of companies said they were willing to trade off little or no functional capabilities in order to gain the benefits of the cloud. And so I think it really does come down to, not technology for technology's sake, but technology that's going to add value. With that said, as you talk to customers, what is it that they're looking for? What is it that they want that the cloud can help offer them?
PAUL: Well, actually, firstly, Jim, I'm actually not surprised at the results of your research. I think at the end of the day, a lot of the benefits that people talk about the cloud, the things like the reduced risk and reduce support needs, they tend to be more IT-centric. And as an engineer, the big challenge is how do I get my job done and out the door? It's not going to help me if when my boss comes to me and says, "That project is running a week late, you need to work at weekends," and particularly that's why people don't want to lose functionality, because if I start losing functionality, then there is a good chance it's going to take me longer to do my job. So really, that means that the challenge for us is, as vendors, is to identify what added value can the cloud bring. Being able to access more capabilities, being able to expand my environment, but more importantly, how can I make, get access to not just software, but data, and I think... As we've gone through the events of this year, and it's been... I guess the best description is it's been challenging. It's been challenging for companies, it's been challenging for employees, and companies have had to adapt to survive. And what we're seeing is obviously remote working became the norm, and depending where you are in the world, I'm here in the UK, remote working is still very much the norm.
PAUL: And inside of NX, we've always supported remote working with NX to be able to give you the software. We have the ability to borrow a license, we have the ability to run virtualized environments and all those types of things. The important thing that we found as people started working, and working with these remote terms, is making sure that people could access their data. And that's where tools like Teamcenter and the new products of the Teamcenter X and Teamcenter Share come in. Being able to make sure that my engineers can access the important information, access their data in a secure environment without actually risking data security. Get to the information they need, without compromising safety and security, and going through and making sure that you've got access to what you need when you need it.
PAUL: That's one of the powers of the cloud that actually helps engineers is that information, as I say, leveraged by tools like Teamcenter, Teamcenter X. There was an aside. What we noticed an interesting phenomenon that started back in March. In March, it seemed like the whole world was entering a period of lockdown, and so people were beginning to do this remote working, we were getting lots of questions about getting set up on remote working. Well, we saw one interesting phenomenon, we actually, from our business records, our business intelligence systems that help as part of our continuous improvement processes, we actually saw that the number of NX sessions and usage of NX increased by 30%. So that just goes to show that people are... That technology is accessible remotely, people are being able to do that and being able to drive that information.
PAUL: We're also seeing this looking forward, that as people are moving more and getting their digitalization strategies in place and moving to leverage the digital twin, the cloud is becoming a key part of that. We're just rolling out a new solution. It combines the power of NX, but also with our Mendix tools, and it allows us to do reporting and analytics. If you think about the digital twin, the whole idea of having a comprehensive digital twin is it gets more and more richer as design develops, as manufacturing develops. So there's a lot of information embedded, not inside of that data, inside of that 3D model. Well, being able to access that from outside of the CAD session, outside of the design session, from any type of device, being able to go on to a tablet device, being able to go on to a phone, drill in.
PAUL: Imagine being like a project manager, and you've got 20 engineers working on a project. Wouldn't it be great if from you from your phone or from your tablet, you can quickly pull up that product, pull up the 3D model, visualize it, and interrogate it, do a check, to see, now, what types of materials are being selected by the design team. Are there any long lead time items in that project that I need to be aware of? That I need to go and talk to procurement for? Going and getting that information, and that's kind of leveraging those types of tools, and the only way you can do that is by leveraging the power of cloud to actually collaborate between the applications and leverage the access that the cloud gives you.
JIM: Thanks, Paul, and it really seems like in a lot of ways, companies that are remote using these tools are actually able to work better together than maybe if they were in the same building trying to join together in a conference room. And I'm going to noodle on your NX Usage increase for a little while, that's got my brain very curious, so I appreciate you sharing that... Sharing that fact. You mentioned earlier, the collaborative virtual reality sessions and things like that, and those to me are more game-changers than things like CAD on the cloud. We've had things like design tool virtualization for quite some time, and those are incredibly valuable, but not necessarily something that is fueling a digital transformation, I would say. How do you see within your products, within the things that you're doing with your customers, how are you helping companies sort of move the bar and really use the cloud to do more? And how are you helping them really fundamentally change the way they work?
PAUL: Yeah, I agree with you. I think that the concept of virtualization has been around a long time. As I say, we've supported it, we have customers, both large and small, running in virtualized environments, and getting benefits from what virtualization brings, but it's not, as you say, it's not really new or game-changing. But what we do see is this need for solutions that support and allow you to work the best way for your business. So, really allowing you to work in the environment you like, adopt cloud technologies where it makes sense, not forced in one way or another. So that brings us to that once again, back to the idea of having the desktop applications and the cloud applications working hand-in-hand, being able to give people the choice of how they deploy solutions without worrying about whether or not... What we're doing and am I going to go down one or other route, that is the wrong dead-end route?
PAUL: And a key element of this is our customers' data. Our customers' data is the important thing. At the end of the day, if something happens and their software gets deleted, their software had, you know... That's no big deal, because at the end of the day, we can always recover that. Their investment, their IP is all in their data. So, it's important for us and as we go forward, and as we evolve in this, is that we make sure that the data that you use, whether you're using cloud solutions or desktop solutions, works, it is the same data. You don't want to have situations where you have to do things to get data into one or other environment, and that's... The important point there, is that we don't want to get to a situation where you have translation.
PAUL: Data translation in any, between different architectures of a system breaks the chain. So digitalization processes then become fragmented, so it's important that the architectures, the structures are the same that work together. And really, that's kinda one of the key elements of what we've been looking at and as we go forward, and using that data, and then using that strategy in our products like Simcenter, using our products inside of Teamcenter, being able to combine these things together to be able to work the best way that suits you. We are, when... I mentioned earlier, we're looking at how can we leverage the cloud to make your life better. We mentioned virtual reality, we mentioned the reporting analytics. We are also looking at specific industries and industry needs and developing combinations of our tools.
PAUL: In our broad Siemens Xcelerator portfolio, we have a wide range of solutions. Being able to combine those solutions are parts of those solutions. Package them up and then offer them on the cloud to complete specific tasks. So for example, something like... In the mold tool business, costing and getting that first quote is a critical for business. If I get my first quote out when I estimate a job, and I get it wildly wrong; too expensive, I'm going to lose the business; too cheap, I'm going to lose money. Well, we've got tools that help you do that. So think about leveraging, tying together design tools like our X-Tools with our Teamcenter costing type tools, with our analysis tools from Simcenter, to package that up into a solution and deliver that.
PAUL: And so we're looking at those types of workflows and saying, "Okay, where can we help?" And we've got a lot of things coming out over the coming years which will go along those. We're not going to talk... We're not going to delve into that, but that gives you those ideas, but also an important thing, of course, is being able to leveraging and allow people to collaborate and manage their data. And our announcement, recent announcements of things like Teamcenter X and Teamcenter Share, Teamcenter X being... Giving the portfolio of Teamcenter solutions in a SaaS/cloud environment. And Teamcenter Share really being targeted at smaller projects, collaboration, small business collaboration, to be able to manage that data. So on all of those things are ways that we are looking to help our customers, but without forcing them down a path. I think this is an important thing, is we want to let them move at their own pace, in their own way, and give them the technologies that underpin their move to these types of technologies.
JIM: Great, thank you, Paul. I really appreciate you sharing your perspective. It's really enlightening, and I look forward to hearing more about future products as the time is right, but thank you again and I hope you stay well.
PAUL: Yeah. Thanks, Jim. Great to talk to you.
[post_title] => Designing on the Cloud Discussion with Siemens (podcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-design-podcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:33:54
[post_modified_gmt] => 2022-12-02 20:33:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9766
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[9] => WP_Post Object
(
[ID] => 9723
[post_author] => 2572
[post_date] => 2020-09-16 11:18:09
[post_date_gmt] => 2020-09-16 15:18:09
[post_content] => Are mobile devices ready for CAD? Is CAD ready for mobile devices? When should you consider use cases for CAD on a tablet?
You can also see related video interviews including: Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell, and Siemens Digital Transformation Progress with Brenda Discher.
Transcript:
JIM: Hi, I'm Jim Brown, President of Tech-Clarity, where we make the business value of technology clear. Today, I'm joined by Paul Brown, a long-time industry leader and Senior Marketing Director at Siemens Digital Industries Software. We're going to talk to Paul about design engineering and how the cloud is really playing a big role there. Let's get some clarity.
JIM: Paul, thanks very much for joining me today.
PAUL: Yeah. Thanks, Jim.
JIM: So we've seen increased interest in the cloud in lots of different kinds of solutions, everything from probably email being run on the cloud for a long time, and ERP and others, but we're starting to see a lot of increased interest in design tools on the cloud and understanding how the process of design and engineering can evolve to leverage capabilities of the cloud. So there are obviously a lot of generic benefits of lower barriers to entry for a solution, whether it be cost or hardware, some lower risk and operational benefits around scalability of users and things along those needs, but we've also noticed that there are some really unique benefits for some engineering solutions, and maybe those come from enhanced collaboration or reach, but they may also come from increased scalability, access to "infinite computing power on demand." When you think about what's happening in design and how the cloud may be changing things, what do you think are the most important things for companies to know?
PAUL: Yeah, it's an interesting point, Jim. And I think we're definitely seeing a lot more interest in cloud-based solutions, but I will say, maybe this is a little bit controversial as a viewpoint, but at the moment, we're seeing a lot of interest, adoption is a bit more steady. I think that there's a lot more kind of people kind of, first of all, looking at what this cloud is going to do for them, so what that's really doing is it's actually getting a number of companies into this area where they've got a level of desktop application and they're starting to use cloud solutions combined together, so you end up with this kind of mixed environment. And really the important thing, and one thing I will say is, that's not a choice that we make or... That is a company choice that is down for the companies as they go forward.
PAUL: We can't, as vendors, force people to one or other approach just because it suits us. Okay, and we really have to look at letting customers decide and work at their own pace and their own way of getting forward in these types of technologies and use what's best for them. And that's always been one of the strategies here at Siemens, is that we've been looking at letting people work this way through and adjust, so the important thing is really is flexibility and being able to access the tools that they need to do their job as they need them and when they need them. I think when you start looking at why people, why the cloud is becoming more interesting, I think design really is, and product development is really a collaborative exercise. There are very few products that we see that are designed by a single person right the way through from start to finish, and so it relies on communicating with other people, sharing design work, and so one of the key elements that we're seeing is people are looking at leveraging the cloud to communicate between groups, to be able to make better design decisions, to share information.
PAUL: An example for you here, about two years ago, we introduced a product called NX Virtual Reality. So NX Virtual Reality first came out, it was great. You're inside your NX session, put on a headset, controllers, and you're immersed inside the product, you're one-to-one scale. We showed it to people. They loved it. I mean, people looked, "Yeah, well, it's... Yeah, it's great." But after a while, they kind of, their response starts coming as like, "Well, yeah, it's good, it gives me some benefits, but you know, yeah, it's okay, but... " There's always a but that comes on. Last year, what we did is we added in the multi-user version of virtual reality, so using multi-user, people can be dispersed around the globe, use a cloud-based solution and join in that session each with their own hardware, each with their own headsets, see each other, be doing work together, being able to measure things, move things, try things out, walk around their design in full-scale, and that's when people started coming back and saying, "Right, now I can do something different in my processes. I can bring people together."
PAUL: And that's because that we're leveraging the power of the cloud to bring together collaboration. I think there are other areas that we're seeing. We're seeing an increasing use of tools like generative engineering, where designers are leveraging the power of simulation and bringing simulation closer into the design process, into the creation process. So leveraging technologies like optimization, and particularly when you start getting into multi-disciplinary optimization, when you are not just looking at structural and stresses, but you're also combining structural and stresses with something like flow and computational fluid dynamics and you're trying to do these really complex type analyses, you need compute power.
PAUL: Now, your options are you have local machines with loads of power or you leverage the cloud, you leverage the compute power in the cloud. As you mentioned, that gives you that kind of near infinite compute power to help you do that and bring that simulation in to help you in that area. So it's giving you that more flexible infrastructure to be able to deliver the power when you need it to the people that need it. And I think those are the types of things that people are looking to actually say, "Okay, well, this is going to make a big difference in my product development process."
JIM: Paul, I really resonate with what you're saying of people looking for things that are not just, "I want to implement the latest technology or use the latest buzz word, but how am I going to change my workflow, how am I going to change my ability to do my job?" And it's interesting, we actually had some survey results along a similar line where we decided to ask companies for the benefits of the cloud, for all of the reduced cost and reduced risk and scalability, etcetera, etcetera, how much functionality are they willing to trade off for that? So basically, whether they're... Are they taking a cloud-first approach or a solution-first approach to their selections. And what we saw really overwhelmingly, the majority of companies said they were willing to trade off little or no functional capabilities in order to gain the benefits of the cloud. And so I think it really does come down to, not technology for technology's sake, but technology that's going to add value. With that said, as you talk to customers, what is it that they're looking for? What is it that they want that the cloud can help offer them?
PAUL: Well, actually, firstly, Jim, I'm actually not surprised at the results of your research. I think at the end of the day, a lot of the benefits that people talk about the cloud, the things like the reduced risk and reduce support needs, they tend to be more IT-centric. And as an engineer, the big challenge is how do I get my job done and out the door? It's not going to help me if when my boss comes to me and says, "That project is running a week late, you need to work at weekends," and particularly that's why people don't want to lose functionality, because if I start losing functionality, then there is a good chance it's going to take me longer to do my job. So really, that means that the challenge for us is, as vendors, is to identify what added value can the cloud bring. Being able to access more capabilities, being able to expand my environment, but more importantly, how can I make, get access to not just software, but data, and I think... As we've gone through the events of this year, and it's been... I guess the best description is it's been challenging. It's been challenging for companies, it's been challenging for employees, and companies have had to adapt to survive. And what we're seeing is obviously remote working became the norm, and depending where you are in the world, I'm here in the UK, remote working is still very much the norm.
PAUL: And inside of NX, we've always supported remote working with NX to be able to give you the software. We have the ability to borrow a license, we have the ability to run virtualized environments and all those types of things. The important thing that we found as people started working, and working with these remote terms, is making sure that people could access their data. And that's where tools like Teamcenter and the new products of the Teamcenter X and Teamcenter Share come in. Being able to make sure that my engineers can access the important information, access their data in a secure environment without actually risking data security. Get to the information they need, without compromising safety and security, and going through and making sure that you've got access to what you need when you need it.
PAUL: That's one of the powers of the cloud that actually helps engineers is that information, as I say, leveraged by tools like Teamcenter, Teamcenter X. There was an aside. What we noticed an interesting phenomenon that started back in March. In March, it seemed like the whole world was entering a period of lockdown, and so people were beginning to do this remote working, we were getting lots of questions about getting set up on remote working. Well, we saw one interesting phenomenon, we actually, from our business records, our business intelligence systems that help as part of our continuous improvement processes, we actually saw that the number of NX sessions and usage of NX increased by 30%. So that just goes to show that people are... That technology is accessible remotely, people are being able to do that and being able to drive that information.
PAUL: We're also seeing this looking forward, that as people are moving more and getting their digitalization strategies in place and moving to leverage the digital twin, the cloud is becoming a key part of that. We're just rolling out a new solution. It combines the power of NX, but also with our Mendix tools, and it allows us to do reporting and analytics. If you think about the digital twin, the whole idea of having a comprehensive digital twin is it gets more and more richer as design develops, as manufacturing develops. So there's a lot of information embedded, not inside of that data, inside of that 3D model. Well, being able to access that from outside of the CAD session, outside of the design session, from any type of device, being able to go on to a tablet device, being able to go on to a phone, drill in.
PAUL: Imagine being like a project manager, and you've got 20 engineers working on a project. Wouldn't it be great if from you from your phone or from your tablet, you can quickly pull up that product, pull up the 3D model, visualize it, and interrogate it, do a check, to see, now, what types of materials are being selected by the design team. Are there any long lead time items in that project that I need to be aware of? That I need to go and talk to procurement for? Going and getting that information, and that's kind of leveraging those types of tools, and the only way you can do that is by leveraging the power of cloud to actually collaborate between the applications and leverage the access that the cloud gives you.
JIM: Thanks, Paul, and it really seems like in a lot of ways, companies that are remote using these tools are actually able to work better together than maybe if they were in the same building trying to join together in a conference room. And I'm going to noodle on your NX Usage increase for a little while, that's got my brain very curious, so I appreciate you sharing that... Sharing that fact. You mentioned earlier, the collaborative virtual reality sessions and things like that, and those to me are more game-changers than things like CAD on the cloud. We've had things like design tool virtualization for quite some time, and those are incredibly valuable, but not necessarily something that is fueling a digital transformation, I would say. How do you see within your products, within the things that you're doing with your customers, how are you helping companies sort of move the bar and really use the cloud to do more? And how are you helping them really fundamentally change the way they work?
PAUL: Yeah, I agree with you. I think that the concept of virtualization has been around a long time. As I say, we've supported it, we have customers, both large and small, running in virtualized environments, and getting benefits from what virtualization brings, but it's not, as you say, it's not really new or game-changing. But what we do see is this need for solutions that support and allow you to work the best way for your business. So, really allowing you to work in the environment you like, adopt cloud technologies where it makes sense, not forced in one way or another. So that brings us to that once again, back to the idea of having the desktop applications and the cloud applications working hand-in-hand, being able to give people the choice of how they deploy solutions without worrying about whether or not... What we're doing and am I going to go down one or other route, that is the wrong dead-end route?
PAUL: And a key element of this is our customers' data. Our customers' data is the important thing. At the end of the day, if something happens and their software gets deleted, their software had, you know... That's no big deal, because at the end of the day, we can always recover that. Their investment, their IP is all in their data. So, it's important for us and as we go forward, and as we evolve in this, is that we make sure that the data that you use, whether you're using cloud solutions or desktop solutions, works, it is the same data. You don't want to have situations where you have to do things to get data into one or other environment, and that's... The important point there, is that we don't want to get to a situation where you have translation.
PAUL: Data translation in any, between different architectures of a system breaks the chain. So digitalization processes then become fragmented, so it's important that the architectures, the structures are the same that work together. And really, that's kinda one of the key elements of what we've been looking at and as we go forward, and using that data, and then using that strategy in our products like Simcenter, using our products inside of Teamcenter, being able to combine these things together to be able to work the best way that suits you. We are, when... I mentioned earlier, we're looking at how can we leverage the cloud to make your life better. We mentioned virtual reality, we mentioned the reporting analytics. We are also looking at specific industries and industry needs and developing combinations of our tools.
PAUL: In our broad Siemens Xcelerator portfolio, we have a wide range of solutions. Being able to combine those solutions are parts of those solutions. Package them up and then offer them on the cloud to complete specific tasks. So for example, something like... In the mold tool business, costing and getting that first quote is a critical for business. If I get my first quote out when I estimate a job, and I get it wildly wrong; too expensive, I'm going to lose the business; too cheap, I'm going to lose money. Well, we've got tools that help you do that. So think about leveraging, tying together design tools like our X-Tools with our Teamcenter costing type tools, with our analysis tools from Simcenter, to package that up into a solution and deliver that.
PAUL: And so we're looking at those types of workflows and saying, "Okay, where can we help?" And we've got a lot of things coming out over the coming years which will go along those. We're not going to talk... We're not going to delve into that, but that gives you those ideas, but also an important thing, of course, is being able to leveraging and allow people to collaborate and manage their data. And our announcement, recent announcements of things like Teamcenter X and Teamcenter Share, Teamcenter X being... Giving the portfolio of Teamcenter solutions in a SaaS/cloud environment. And Teamcenter Share really being targeted at smaller projects, collaboration, small business collaboration, to be able to manage that data. So on all of those things are ways that we are looking to help our customers, but without forcing them down a path. I think this is an important thing, is we want to let them move at their own pace, in their own way, and give them the technologies that underpin their move to these types of technologies.
JIM: Great, thank you, Paul. I really appreciate you sharing your perspective. It's really enlightening, and I look forward to hearing more about future products as the time is right, but thank you again and I hope you stay well.
PAUL: Yeah. Thanks, Jim. Great to talk to you.
[post_title] => Designing on the Cloud Discussion with Siemens (podcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-design-podcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:33:54
[post_modified_gmt] => 2022-12-02 20:33:54
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9766
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[9] => WP_Post Object
(
[ID] => 9723
[post_author] => 2572
[post_date] => 2020-09-16 11:18:09
[post_date_gmt] => 2020-09-16 15:18:09
[post_content] => Are mobile devices ready for CAD? Is CAD ready for mobile devices? When should you consider use cases for CAD on a tablet?
 CAD tools have evolved significantly over the last several decades, which has coincided with several platform shifts. With the advancements in tablets combined with evolutions in CAD, we may be at the dawn of the latest shift. Together, CAD on a tablet can offer many benefits. The combination can expand the use of CAD with greater accessibility and flexibility, improved productivity, and better customer service. This blog post discusses why that is and reveals several examples of how CAD on a tablet can benefit a company, especially smaller companies and start-ups.
Read the full guest post on the Shapr3D blog.
[post_title] => How does CAD on mobile devices help?
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => mobile-devices-for-cad
[to_ping] =>
[pinged] =>
[post_modified] => 2025-08-13 19:13:15
[post_modified_gmt] => 2025-08-13 23:13:15
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9723
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[10] => WP_Post Object
(
[ID] => 9661
[post_author] => 2
[post_date] => 2020-09-04 13:40:51
[post_date_gmt] => 2020-09-04 17:40:51
[post_content] =>
CAD tools have evolved significantly over the last several decades, which has coincided with several platform shifts. With the advancements in tablets combined with evolutions in CAD, we may be at the dawn of the latest shift. Together, CAD on a tablet can offer many benefits. The combination can expand the use of CAD with greater accessibility and flexibility, improved productivity, and better customer service. This blog post discusses why that is and reveals several examples of how CAD on a tablet can benefit a company, especially smaller companies and start-ups.
Read the full guest post on the Shapr3D blog.
[post_title] => How does CAD on mobile devices help?
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => mobile-devices-for-cad
[to_ping] =>
[pinged] =>
[post_modified] => 2025-08-13 19:13:15
[post_modified_gmt] => 2025-08-13 23:13:15
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9723
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[10] => WP_Post Object
(
[ID] => 9661
[post_author] => 2
[post_date] => 2020-09-04 13:40:51
[post_date_gmt] => 2020-09-04 17:40:51
[post_content] =>  How has digital transformation progressed for Siemens' customers and how are they leveraging Siemens' cloud technologies to fuel their digital initiatives? How have Siemens' customers with a digital mindset faired differently than others during the COVID-19 disruption? Tech-Clarity's Jim Brown got together (virtually) with Siemens Digital Industries Software EVP Bob Jones to share perspectives and case studies.
[audio mp3="https://tech-clarity.com/wp-content/uploads/2020/09/Tech-Clarity-TV-Cloud-Interview-Bob-Jones-09-03.mp3"][/audio]
Get more information about Siemens Cloud Solutions from Siemens Digital Industries Software.
You can also see related video interviews including a Cloud Progress Report with Bill Boswell and Siemens Digital Transformation Progress with Brenda Discher.
Transcript:
JIM: Hi, I'm Jim Brown, president of Tech Clarity, where we make the business value of technology clear. Today, I'm checking back in with Bob Jones, Senior Vice President at Siemens Digital Industries Software. I spoke with Bob a little over a year ago to gain a customer's perspective on how Siemens is helping their customers move to the cloud. And we're going to check back in to really understand how has the digital transformation of Siemens' customers progressed and how are they leveraging Siemens' technologies in the cloud to really fuel their digital transformation. How's that been going? Let's get some clarity.
JIM: Bob, thank you for joining me again today. Unfortunately, we're doing this remotely ... trying to keep everybody safe because of COVID. And in fact, due to some additional challenges in being distanced we're doing this audio only instead of video. But it's always great to get in touch with you and to really understand what you're experiencing. So thanks for joining today.
BOB: Glad to be able to join you Jim. Thank you.
JIM: I know you speak a lot with your clients, not just about technology, but about their business strategies and what they're really trying to accomplish. But a lot has change for both Siemens and for your customers since we last talked more than a year ago. Then obviously, things have changed even more dramatically over the last few months due to the global pandemic. Maybe you can give us a little bit of an idea of what have company's been asking for? How has that changed as they've gotten more mature with digitalization and moving to the cloud, but also because of COVID?
BOB: Thank you, Jim. You mentioned already COVID has been a great impact, a significant impact on our customers, it's really definitely accelerated their move to digitalization. And I guess what I would tell you is what we've learned from customers in the last 12 to 18 months is that it's not just about this concept of digital transformation. What we've seen from our customers is those that have had this digital mindset, which really means that they are the pioneers, they are the ones that are willing to drive digitalization throughout their organization and really look at how they can leverage cloud to improve their overall innovation process, those customers have really achieved some incredible success. And I would go as far as to say is that those customers with the digital mindset, they've actually been able to transition or make that transition to a remote working environment or work from home as a result of COVID much more seamlessly than those customers or companies that did not have that digital mindset.
BOB: And in fact, I would argue that the ones that didn't have a digital mindset, they've quite honestly been exposed as a result of COVID and they've struggled because of that. And when I think about the impact of COVID, I think that it's also very evident when we think about our customers in the SMB space, because they have the same need to leverage better collaboration and capabilities that's enabled to the cloud, but what they don't have is the personnel or the financial resources to make that jump as fast as some of the larger companies could. And I think an example of a SMB that had that digital mindset and thrived because of it, is Rurok Industries. Now, Rurok is a small mountain bike manufacturer, they're a startup, but ironically, even though they're a startup, they still have a global footprint because they have design centers here in the US, in Las Vegas, they have a design center in the Philippines, they're manufacturing over in Taiwan, and they have customers essentially throughout the world.
BOB: So Rurok is a classic problem of a startup trying to thrive in this global economy while dealing with the challenges of COVID. Now, Rurok had recently selected Solid Edge and deployed Solid Edge as their overall global design system, but because of COVID, they now faced a new challenge and that they needed to be able to remain competitive and create and design these next generation mountain bikes while protecting all their employees through travel restrictions and isolation guidelines. And what Rurok... They benefited from this, is they leveraged our Teamcenter Share technology, they were actually a pilot customer of Teamcenter Share, and it gave them instant capability to enable design collaboration around the globe and allow them to continue to compete and drive down their overall innovation life cycle to produce next generation mountain bikes. In fact, their CEO, PJ Tolentino and his team have a great video summary of how they're leveraging Teamcenter and Solid Edge in the cloud to drive this collaboration and able to even leverage advanced capabilities such as augmented reality to support their design collaboration process.
BOB: If I think further beyond COVID, I'd also say that something new we're hearing from customers, I would classify as “instant-on.” What this means is, yes, our customers want all the benefits associated to the cloud; lower cost, improved collaboration, and agility. But they're also saying it's not enough just to have that technology generically, they need technology delivered to them that enables the best practices within their industries so they can have an immediate impact on their overall productivity. And that essentially is one of the driving forces behind what we recently launched called Teamcenter X. It's a cloud-based, instant capability for PLM that leverages all the robustness of Teamcenter. But more importantly, we pre-configured it with some best practices around design, collaboration, document management, raw material management, change requirements, and other capabilities that our customers need to drive their innovation process. And I would summarize it to say, think of Rapid Start, Teamcenter Rapid Start with all the benefits of the cloud.
BOB: And lastly, I'd say it's not enough to give our customers that instant-on access to industry best practices, but also be able to understand through telemetry, what some best practices are within their own organization, and then allow that to be somewhat codified so that new users can immediately adopt the best practices of the industry as well as best practices within their own organization. And then lastly, I would also say that - something we continue to hear that's more consistent with what we've heard previously - is this concept of flexibility. That is what we refer to as “your way, your pace.” Our customers continue to tell us that they need to move to the cloud, they need to move the digitalization, but they need the ability to be flexible in how they do that. Whether it's the leverage their existing infrastructure, but purchase our software in a different mode, or whether they need to manage services capability, or whether they want to go to a full SaaS technology model as well as SaaS business model. So it's this concept that’s given them the flexibility to leverage their existing investments, their existing best practices, while adopting the cloud to drive the benefit in the area that it's most needed.
JIM: Great. Thanks, Bob. It's interesting, our research is also showing some indications that companies are accelerating their digital transformation based on the impact of COVID. And it's certainly a way that companies are looking to survive and maybe even leapfrog a little bit.
JIM: Your role spans really from early conversations with potential customers through some of the longer-term relationships you've had with existing customers and understanding how they gain value from your solutions. Digitalization is such a broad concept. As companies digitally transform, it could be anything from streamlining a design workflow to developing a robust digital twin with the connectivity to actuals - the sensors on the twin itself - and bringing in enterprise information to make improvements in the plant or service. So there's just so many different kinds of initiatives that companies can take. What are you seeing as some of the things that your customers have achieved through digital transformation and the use of the cloud?
BOB: Thank you, Jim. That's an excellent question. I guess there's two or three examples that come immediately to mind. And I'll start with a company that most people probably don't associate with PLM, and that's Land O'Lakes. Now, interestingly enough, Land O'Lakes is a very well-known company, mostly here in the US, and they are celebrating their 100-year anniversary next year. And Land O'Lakes, I think, faces the same challenge that I see across every industry. That is that they have a backlog of demand on their IT organization. They have more IT projects than they can effectively deliver in a reasonable time. So, Land O'Lakes took a decision that they did not want to outsource this because they recognized this was a critical differentiator for them. Rather they wanted to bring that innovation back in house.
BOB: So what Land O'Lakes decided to do was partner with us to leverage our Mendix technology, our cloud native low-code application development platform. They wanted to leverage that not just so their own IT professionals could code the capabilities that their business users needed, but rather so that IT could orchestrate innovation across their business, across their ecosystem. So today, Land O'Lakes is using Mendix to drive and enable very personalized online portals that support over 350,000 farmers, distributors, retailers, and wholesalers. And they're doing this in a way that helps them achieve their goals for overall sustainability but more importantly to drive a much more effective supply chain.
BOB: Now, I use that example because I think it also points out something that is a trend that we see emerging and we believe will become very relevant in the future, and that is that the employees of our customers are no longer just consumers of our technology. The employees of our customers are increasingly moving to be the shapers and even the developers of our technology and tailoring it to the specific needs of their company. And I think that's an important trait that's going to ultimately reshape our industry in many ways. That's one example.
BOB: A second example is a company called Vyaire, they're a medical device company. What's interesting about them is they were recently spun out of a much larger organization, but as part of their spin-out they needed to build from scratch their overall PLM environment. So they partnered with us, leveraging Teamcenter and leveraging our cloud delivery of Teamcenter, so that essentially they are able to deploy a PLM solution across their enterprise, drive adoption and enablement in their users, and reach a level of productivity all within three months. That's, I think, an excellent example of what the cloud can provide in terms of agility and speed to enterprises of all size.
BOB: And then the last example that I can think of is from the aerospace industry. Now, unfortunately, I don't have permission yet to share this particular company's name, but it's a great example of how they've had to leverage a broad portfolio of simulation design and data management collaboration and do that leveraging the cloud so that they can still drive this collaboration while everybody works remotely.
BOB: Now, this particular company is designing an electric aircraft for general aviation, and they identified a serious problem during some of their virtual prove-outs, and that they needed to improve the overall ingress, the access for a pilot to get into the cockpit. And ultimately that meant they needed to stretch that aircraft a few inches to meet the regulations for ingress. Now, if we think about the impact that has to an overall aircraft in terms of its performance characteristics, of its aerodynamics, and the amount of design and simulation and analysis whether they'd be for static loads, overall performance, fuel consumption, it's quite a large task.
BOB: Now this company, because they're able to continue to design and collaborate and do all this while working remotely, completed that entire design change and validation of that design change in just seven days. I think that's a tremendous example of what customers can accomplish by leveraging both the integrated portfolio of capabilities across their innovation lifecycle, and also leveraging the cloud that enables them to do that, whether it's to work from home or whether it's to enable overall global collaboration.
JIM: Yeah, I know. We talked before about how companies would really be changing the way they're working, and certainly companies have changed things pretty dramatically. Those are great examples, Bob. I really appreciate it.
Thank you for joining me today. It's always a pleasure. I really enjoy sharing with your experiences and what you're seeing your customers be able to do with your technologies and with your services. So thank you so much for joining, and please stay well.
BOB: Yeah, and Jim, thank you for the opportunity to share some of these examples with you and your listeners.
[post_title] => Digital Transformation at Siemens' Customers (podcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => digital-transformation-customers
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:34:29
[post_modified_gmt] => 2022-12-02 20:34:29
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9661
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[11] => WP_Post Object
(
[ID] => 9629
[post_author] => 2
[post_date] => 2020-09-02 12:42:54
[post_date_gmt] => 2020-09-02 16:42:54
[post_content] =>
How has digital transformation progressed for Siemens' customers and how are they leveraging Siemens' cloud technologies to fuel their digital initiatives? How have Siemens' customers with a digital mindset faired differently than others during the COVID-19 disruption? Tech-Clarity's Jim Brown got together (virtually) with Siemens Digital Industries Software EVP Bob Jones to share perspectives and case studies.
[audio mp3="https://tech-clarity.com/wp-content/uploads/2020/09/Tech-Clarity-TV-Cloud-Interview-Bob-Jones-09-03.mp3"][/audio]
Get more information about Siemens Cloud Solutions from Siemens Digital Industries Software.
You can also see related video interviews including a Cloud Progress Report with Bill Boswell and Siemens Digital Transformation Progress with Brenda Discher.
Transcript:
JIM: Hi, I'm Jim Brown, president of Tech Clarity, where we make the business value of technology clear. Today, I'm checking back in with Bob Jones, Senior Vice President at Siemens Digital Industries Software. I spoke with Bob a little over a year ago to gain a customer's perspective on how Siemens is helping their customers move to the cloud. And we're going to check back in to really understand how has the digital transformation of Siemens' customers progressed and how are they leveraging Siemens' technologies in the cloud to really fuel their digital transformation. How's that been going? Let's get some clarity.
JIM: Bob, thank you for joining me again today. Unfortunately, we're doing this remotely ... trying to keep everybody safe because of COVID. And in fact, due to some additional challenges in being distanced we're doing this audio only instead of video. But it's always great to get in touch with you and to really understand what you're experiencing. So thanks for joining today.
BOB: Glad to be able to join you Jim. Thank you.
JIM: I know you speak a lot with your clients, not just about technology, but about their business strategies and what they're really trying to accomplish. But a lot has change for both Siemens and for your customers since we last talked more than a year ago. Then obviously, things have changed even more dramatically over the last few months due to the global pandemic. Maybe you can give us a little bit of an idea of what have company's been asking for? How has that changed as they've gotten more mature with digitalization and moving to the cloud, but also because of COVID?
BOB: Thank you, Jim. You mentioned already COVID has been a great impact, a significant impact on our customers, it's really definitely accelerated their move to digitalization. And I guess what I would tell you is what we've learned from customers in the last 12 to 18 months is that it's not just about this concept of digital transformation. What we've seen from our customers is those that have had this digital mindset, which really means that they are the pioneers, they are the ones that are willing to drive digitalization throughout their organization and really look at how they can leverage cloud to improve their overall innovation process, those customers have really achieved some incredible success. And I would go as far as to say is that those customers with the digital mindset, they've actually been able to transition or make that transition to a remote working environment or work from home as a result of COVID much more seamlessly than those customers or companies that did not have that digital mindset.
BOB: And in fact, I would argue that the ones that didn't have a digital mindset, they've quite honestly been exposed as a result of COVID and they've struggled because of that. And when I think about the impact of COVID, I think that it's also very evident when we think about our customers in the SMB space, because they have the same need to leverage better collaboration and capabilities that's enabled to the cloud, but what they don't have is the personnel or the financial resources to make that jump as fast as some of the larger companies could. And I think an example of a SMB that had that digital mindset and thrived because of it, is Rurok Industries. Now, Rurok is a small mountain bike manufacturer, they're a startup, but ironically, even though they're a startup, they still have a global footprint because they have design centers here in the US, in Las Vegas, they have a design center in the Philippines, they're manufacturing over in Taiwan, and they have customers essentially throughout the world.
BOB: So Rurok is a classic problem of a startup trying to thrive in this global economy while dealing with the challenges of COVID. Now, Rurok had recently selected Solid Edge and deployed Solid Edge as their overall global design system, but because of COVID, they now faced a new challenge and that they needed to be able to remain competitive and create and design these next generation mountain bikes while protecting all their employees through travel restrictions and isolation guidelines. And what Rurok... They benefited from this, is they leveraged our Teamcenter Share technology, they were actually a pilot customer of Teamcenter Share, and it gave them instant capability to enable design collaboration around the globe and allow them to continue to compete and drive down their overall innovation life cycle to produce next generation mountain bikes. In fact, their CEO, PJ Tolentino and his team have a great video summary of how they're leveraging Teamcenter and Solid Edge in the cloud to drive this collaboration and able to even leverage advanced capabilities such as augmented reality to support their design collaboration process.
BOB: If I think further beyond COVID, I'd also say that something new we're hearing from customers, I would classify as “instant-on.” What this means is, yes, our customers want all the benefits associated to the cloud; lower cost, improved collaboration, and agility. But they're also saying it's not enough just to have that technology generically, they need technology delivered to them that enables the best practices within their industries so they can have an immediate impact on their overall productivity. And that essentially is one of the driving forces behind what we recently launched called Teamcenter X. It's a cloud-based, instant capability for PLM that leverages all the robustness of Teamcenter. But more importantly, we pre-configured it with some best practices around design, collaboration, document management, raw material management, change requirements, and other capabilities that our customers need to drive their innovation process. And I would summarize it to say, think of Rapid Start, Teamcenter Rapid Start with all the benefits of the cloud.
BOB: And lastly, I'd say it's not enough to give our customers that instant-on access to industry best practices, but also be able to understand through telemetry, what some best practices are within their own organization, and then allow that to be somewhat codified so that new users can immediately adopt the best practices of the industry as well as best practices within their own organization. And then lastly, I would also say that - something we continue to hear that's more consistent with what we've heard previously - is this concept of flexibility. That is what we refer to as “your way, your pace.” Our customers continue to tell us that they need to move to the cloud, they need to move the digitalization, but they need the ability to be flexible in how they do that. Whether it's the leverage their existing infrastructure, but purchase our software in a different mode, or whether they need to manage services capability, or whether they want to go to a full SaaS technology model as well as SaaS business model. So it's this concept that’s given them the flexibility to leverage their existing investments, their existing best practices, while adopting the cloud to drive the benefit in the area that it's most needed.
JIM: Great. Thanks, Bob. It's interesting, our research is also showing some indications that companies are accelerating their digital transformation based on the impact of COVID. And it's certainly a way that companies are looking to survive and maybe even leapfrog a little bit.
JIM: Your role spans really from early conversations with potential customers through some of the longer-term relationships you've had with existing customers and understanding how they gain value from your solutions. Digitalization is such a broad concept. As companies digitally transform, it could be anything from streamlining a design workflow to developing a robust digital twin with the connectivity to actuals - the sensors on the twin itself - and bringing in enterprise information to make improvements in the plant or service. So there's just so many different kinds of initiatives that companies can take. What are you seeing as some of the things that your customers have achieved through digital transformation and the use of the cloud?
BOB: Thank you, Jim. That's an excellent question. I guess there's two or three examples that come immediately to mind. And I'll start with a company that most people probably don't associate with PLM, and that's Land O'Lakes. Now, interestingly enough, Land O'Lakes is a very well-known company, mostly here in the US, and they are celebrating their 100-year anniversary next year. And Land O'Lakes, I think, faces the same challenge that I see across every industry. That is that they have a backlog of demand on their IT organization. They have more IT projects than they can effectively deliver in a reasonable time. So, Land O'Lakes took a decision that they did not want to outsource this because they recognized this was a critical differentiator for them. Rather they wanted to bring that innovation back in house.
BOB: So what Land O'Lakes decided to do was partner with us to leverage our Mendix technology, our cloud native low-code application development platform. They wanted to leverage that not just so their own IT professionals could code the capabilities that their business users needed, but rather so that IT could orchestrate innovation across their business, across their ecosystem. So today, Land O'Lakes is using Mendix to drive and enable very personalized online portals that support over 350,000 farmers, distributors, retailers, and wholesalers. And they're doing this in a way that helps them achieve their goals for overall sustainability but more importantly to drive a much more effective supply chain.
BOB: Now, I use that example because I think it also points out something that is a trend that we see emerging and we believe will become very relevant in the future, and that is that the employees of our customers are no longer just consumers of our technology. The employees of our customers are increasingly moving to be the shapers and even the developers of our technology and tailoring it to the specific needs of their company. And I think that's an important trait that's going to ultimately reshape our industry in many ways. That's one example.
BOB: A second example is a company called Vyaire, they're a medical device company. What's interesting about them is they were recently spun out of a much larger organization, but as part of their spin-out they needed to build from scratch their overall PLM environment. So they partnered with us, leveraging Teamcenter and leveraging our cloud delivery of Teamcenter, so that essentially they are able to deploy a PLM solution across their enterprise, drive adoption and enablement in their users, and reach a level of productivity all within three months. That's, I think, an excellent example of what the cloud can provide in terms of agility and speed to enterprises of all size.
BOB: And then the last example that I can think of is from the aerospace industry. Now, unfortunately, I don't have permission yet to share this particular company's name, but it's a great example of how they've had to leverage a broad portfolio of simulation design and data management collaboration and do that leveraging the cloud so that they can still drive this collaboration while everybody works remotely.
BOB: Now, this particular company is designing an electric aircraft for general aviation, and they identified a serious problem during some of their virtual prove-outs, and that they needed to improve the overall ingress, the access for a pilot to get into the cockpit. And ultimately that meant they needed to stretch that aircraft a few inches to meet the regulations for ingress. Now, if we think about the impact that has to an overall aircraft in terms of its performance characteristics, of its aerodynamics, and the amount of design and simulation and analysis whether they'd be for static loads, overall performance, fuel consumption, it's quite a large task.
BOB: Now this company, because they're able to continue to design and collaborate and do all this while working remotely, completed that entire design change and validation of that design change in just seven days. I think that's a tremendous example of what customers can accomplish by leveraging both the integrated portfolio of capabilities across their innovation lifecycle, and also leveraging the cloud that enables them to do that, whether it's to work from home or whether it's to enable overall global collaboration.
JIM: Yeah, I know. We talked before about how companies would really be changing the way they're working, and certainly companies have changed things pretty dramatically. Those are great examples, Bob. I really appreciate it.
Thank you for joining me today. It's always a pleasure. I really enjoy sharing with your experiences and what you're seeing your customers be able to do with your technologies and with your services. So thank you so much for joining, and please stay well.
BOB: Yeah, and Jim, thank you for the opportunity to share some of these examples with you and your listeners.
[post_title] => Digital Transformation at Siemens' Customers (podcast)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => digital-transformation-customers
[to_ping] =>
[pinged] =>
[post_modified] => 2022-12-02 15:34:29
[post_modified_gmt] => 2022-12-02 20:34:29
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9661
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[11] => WP_Post Object
(
[ID] => 9629
[post_author] => 2
[post_date] => 2020-09-02 12:42:54
[post_date_gmt] => 2020-09-02 16:42:54
[post_content] =>  How are companies adjusting their strategies to survive COVID-19 disruption without losing sight of long-term business sustainability? Read our survey of over 190 companies to find out how they are prioritizing limited resources to maintain focus on success factors including:
How are companies adjusting their strategies to survive COVID-19 disruption without losing sight of long-term business sustainability? Read our survey of over 190 companies to find out how they are prioritizing limited resources to maintain focus on success factors including:
- Addressing changing business models
- Adopting new technology
- Protecting the environment
Table of Contents
- Global Disruptions Compound Business Risk
- Disruption Impacts Company Strategies
- Strategies Understandably Reflect Disconnects
- COVID has Multi-Faceted Impacts
- Recognizing What's Important to Long-Term Success
- Factors Influencing Strategy are Relatively Consistent
- Companies Simply Can't Do Everything
- Business Transformation Leads Sustainability Needs
- Workforce Development Importance Has Increased
- Conclusions and Next Steps
- About the Research
- Acknowledgments
Global Disruptions Compound Business Risk
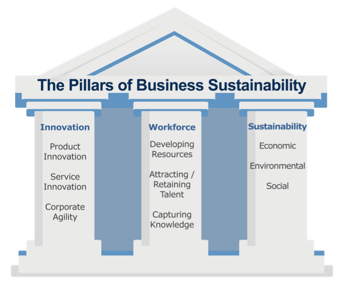 Recognizing Turbulent Times
Recognizing Turbulent Times
Business Transformation Leads Sustainability Needs
Being Aware that Economics are Still the Largest Drivers of SuccessConclusions and Next Steps
Companies are in Crisis due to COVID ware capabilities will help you improve how you integrate mechanical, electronics, and electrical designs?
Tech-Clarity’s buyer’s guide, How to Select the Ideal Solution for Today’s Smart Products: Buyer’s Guide for Electrical Design, explores this question. As companies strive to make modern products smarter, innovative and more affordable, the integration of mechanical, electrical and electronic systems has become critical. Unfortunately mechanical and electrical engineers speak different languages, use separate tools, follow different design approaches and have inherent knowledge silos. Companies that have solutions to overcome these challenges will have a significant advantage in the global economy.
Please enjoy the summary* below. Please visit our sponsor, Siemens, for the full research (registration required).
ware capabilities will help you improve how you integrate mechanical, electronics, and electrical designs?
Tech-Clarity’s buyer’s guide, How to Select the Ideal Solution for Today’s Smart Products: Buyer’s Guide for Electrical Design, explores this question. As companies strive to make modern products smarter, innovative and more affordable, the integration of mechanical, electrical and electronic systems has become critical. Unfortunately mechanical and electrical engineers speak different languages, use separate tools, follow different design approaches and have inherent knowledge silos. Companies that have solutions to overcome these challenges will have a significant advantage in the global economy.
Please enjoy the summary* below. Please visit our sponsor, Siemens, for the full research (registration required).
Table of Contents
- Executive Overview
- The Growing Importance of Electrical Design in Product Development
- Address the Challenges of Electrical Design
- Define the Electrical Distribution System
- Engineer Wiring and Harness Design in the Context of the Complete Product
- Plan Electrical Routing
- Prepare for Manufacturing
- Service Requirements
- Vendor Requirements
- Identify Unique Company Needs
- Conclusion
- Recommendations
- About the Author
- Acknowledgments
Executive Overview
Today’s modern products are rarely purely mechanical. Companies rely on electrical and electronic systems more than ever to make products smarter, add innovation, and lower cost. As such, electrical design has become increasingly critical to product development. However, to be successful, mechanical and electrical designs must seamlessly integrate. The problem is that mechanical and electrical engineers speak different languages, use separate tools, and follow unique development approaches. To make matters worse, there are inherent knowledge silos across the two domains. Ignoring these challenges and not finding solutions to break down barriers and bring the teams closer can come at a significant cost to the company. Tech-Clarity research [1] finds collaboration challenges results in:
To make matters worse, there are inherent knowledge silos across the two domains. Ignoring these challenges and not finding solutions to break down barriers and bring the teams closer can come at a significant cost to the company. Tech-Clarity research [1] finds collaboration challenges results in:
- Higher cost
- Market delays
- Missed customer expectations
- Lost revenue opportunities
- Poor quality
About This Guide
This buyer’s guide explores the capabilities needed in a complete electrical design solution. It consists of four major sections covering software tool functionality, service requirements, vendor attributes, and unique company considerations for a complete solution to support electrical design, especially in the context of developing the entire product (Figure 1). Each section includes a checklist of key requirements to consider when selecting a solution.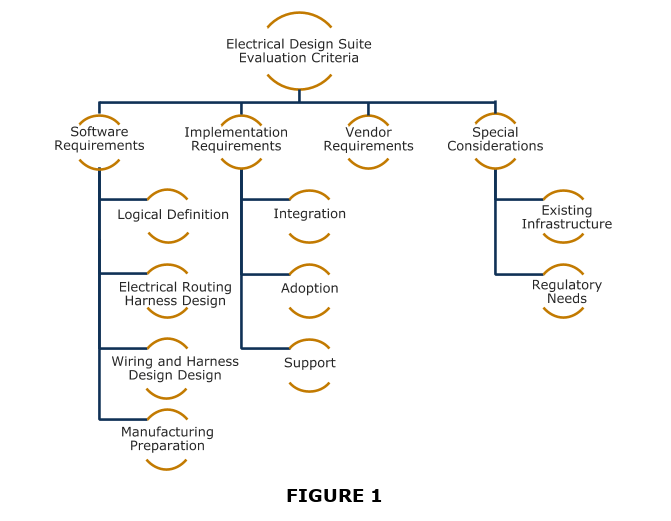 This guide is not an all-encompassing requirements list. It provides a high-level overview. PCB Design is also an important component of electrical and electronic systems design, but for the purposes of this guide, it was considered out of scope. For more information on a complete integrated product development suite beyond electrical design, read the Tech-Clarity report, “Buyer’s Guide for Engineers: How to Select Essential Tools for Product Design.”
This guide is not an all-encompassing requirements list. It provides a high-level overview. PCB Design is also an important component of electrical and electronic systems design, but for the purposes of this guide, it was considered out of scope. For more information on a complete integrated product development suite beyond electrical design, read the Tech-Clarity report, “Buyer’s Guide for Engineers: How to Select Essential Tools for Product Design.”
Recommendations
Based on industry experience and research for this report, Tech-Clarity offers the following recommendations:- Create a digital thread across your development process with an end-to-end integrated product development suite that includes electrical design.
- Consider solutions that support defining the electrical distribution system, wiring and harness design, electrical routing, and preparing for
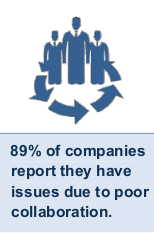 manufacturing.
manufacturing. - Support collaboration across mechanical and electrical engineers. Wiring and harness design should be done in the context of the entire product and exchanging ECAD / MCAD data should be automated.
- Ensure you have traceability and changes automatically update across the entire product, including the electrical design.
- Use an electrical design solution that will support the development of a single high-fidelity model to support your digitalization strategy and create a single source of truth.
- Ensure your solution will have the support behind it to make it a success at your company.
- Select a vendor who will be a good partner.
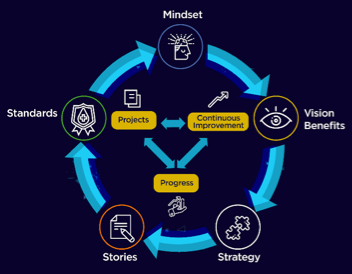 What does effective storytelling have to do with succeeding with Industry 4.0? Plenty.
What does effective storytelling have to do with succeeding with Industry 4.0? Plenty.
 [post_title] => Succeeding with Industry 4.0: Understanding the Whole Elephant (article)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => succeeding-with-industry4-0
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:56
[post_modified_gmt] => 2022-11-15 03:25:56
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9537
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[14] => WP_Post Object
(
[ID] => 9581
[post_author] => 2572
[post_date] => 2020-08-26 14:07:03
[post_date_gmt] => 2020-08-26 18:07:03
[post_content] =>
[post_title] => Succeeding with Industry 4.0: Understanding the Whole Elephant (article)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => succeeding-with-industry4-0
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:56
[post_modified_gmt] => 2022-11-15 03:25:56
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9537
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[14] => WP_Post Object
(
[ID] => 9581
[post_author] => 2572
[post_date] => 2020-08-26 14:07:03
[post_date_gmt] => 2020-08-26 18:07:03
[post_content] =>  How can schools best prepare today's STEM students to close the engineering skills gap so that new graduates are ready for the real world?
Tech-Clarity’s research report, “How Academia Can Close the Engineering Skills Gap in the Age of Digitalization” explores this question. This research shares a global perspective on what colleges and universities are doing to develop the engineering talent needed in the age of digitalization. It also examines the impact COVID-19 has had on instructions and offers lessons learned that can be used to improve the educational experience in the Fall, as well as in the future.
Please enjoy the summary* below. For the full research, please visit our sponsor, Siemens (registration required).
How can schools best prepare today's STEM students to close the engineering skills gap so that new graduates are ready for the real world?
Tech-Clarity’s research report, “How Academia Can Close the Engineering Skills Gap in the Age of Digitalization” explores this question. This research shares a global perspective on what colleges and universities are doing to develop the engineering talent needed in the age of digitalization. It also examines the impact COVID-19 has had on instructions and offers lessons learned that can be used to improve the educational experience in the Fall, as well as in the future.
Please enjoy the summary* below. For the full research, please visit our sponsor, Siemens (registration required).
Table of Contents
- Executive Summary
- Meet Industry Needs
- Embrace Digitalization
- Prepare for the New Corporate Culture
- Opportunities for Improvement
- Adapt to the Needs of the 2020s
- 1. Offer Long Term, Realistic Projects
- 2. Reshape the Curriculum
- 3. Leverage Software
- 4. Enhance Learning with Digital Twins
- 5. Enrich Learning with Technology
- 6. Develop Collaboration Skills
- 7. Prepare for Cross-Functional Horizontal Teams
- 8. Build Resumes and Offer Career Insight
- 9. Partner with Industry for Technology Thought Leadership
- Conclusions
- Recommendations
- Acknowledgments
Executive Summary
 Requirements for New Engineers
Industry needs more engineering graduates. New engineers must be comfortable with cross discipline projects, a range of technology including digitalization, and horizontal organizations. Along with this deep skillset, industry wants engineers to be strong problem solvers with the aptitude to apply technology to solve problems. Previous Tech-Clarity research finds that industry believes schools could do better to meet these requirements.
Requirements for Learning Approaches
Much of this is due to the traditional lecture-based approach to education. By reshaping the curriculum to focus on project-based learning and partnering with industry thought leaders, there is tremendous opportunity to improve students’ education.
Requirements for New Engineers
Industry needs more engineering graduates. New engineers must be comfortable with cross discipline projects, a range of technology including digitalization, and horizontal organizations. Along with this deep skillset, industry wants engineers to be strong problem solvers with the aptitude to apply technology to solve problems. Previous Tech-Clarity research finds that industry believes schools could do better to meet these requirements.
Requirements for Learning Approaches
Much of this is due to the traditional lecture-based approach to education. By reshaping the curriculum to focus on project-based learning and partnering with industry thought leaders, there is tremendous opportunity to improve students’ education.
This Research
This research builds upon past Tech-Clarity research, Close the Engineering Skills Gap when over two hundred companies were surveyed to identify specific skills industry wants to see in graduating engineers. For this new research, educators from around the world were interviewed to understand what schools are doing to better prepare their students. This report details different perspectives and shares advice to evolve the curriculum to meet today’s needs. COVID-19 The research explores how some schools have embraced digitalization, incorporated the latest technology trends, and leveraged industry partnerships. It also shares lessons learned as a result of the COVID-19 pandemic. Schools should:
Schools should:
- Offer long term realistic projects
- Reshape the curriculum
- Leverage software
- Enhance learning with digital twins
- Enrich learning with technology
- Develop collaboration skills
- Prepare for horizontal cross-functional teams
- Build resumes and offer career insight
- Partner with industry for technology thought leadership
Recommendations
Next Steps
 Based on this research and our experience, Tech-Clarity offers the following recommendations:
Based on this research and our experience, Tech-Clarity offers the following recommendations:
-
Restructure academic programs to maximize project-based learning opportunities. This can be done as part of the regular curriculum or as extracurricular actives.
-
Recognize the numerous benefits of project-based learning for both students and industry. Design programs to ensure that participants realize these benefits.
-
Involve industry to provide real-world problems as well as mentorships and internships to ensure students have the opportunity to learn from their experiences and expertise.
-
Projects need to develop problem-solving and collaboration skills, while exposing engineers to multi-discipline work
-
Reshape the curriculum and remove content that is less valuable for today’s engineers.
-
Bring in industry thought leaders and subject matter experts into the classroom.
 What should supply chain-centric innovators consider when looking for PLM? How has the PLM landscape changed over the last several years, and how does SaaS fit into that picture?
Please enjoy the summary* below. For the full research, please visit our sponsor, Upchain (registration required).
What should supply chain-centric innovators consider when looking for PLM? How has the PLM landscape changed over the last several years, and how does SaaS fit into that picture?
Please enjoy the summary* below. For the full research, please visit our sponsor, Upchain (registration required).
Table of Contents
- The State of Cloud PLM
- Why Cloud SaaS is so Compelling
- Current Times Increase Cloud Appeal
- Choosing the Right PLM
- The Shifting Cloud versus Capabilities Tradeoff
- Recommendations and Next Steps
- Acknowledgments
The State of Cloud PLM
Significant Benefits of the Cloud "Cloud PLM" is not a Cookie Cutter Decision
"Cloud PLM" is not a Cookie Cutter Decision
Recommendations and Next Steps
Investigate SaaS Solutions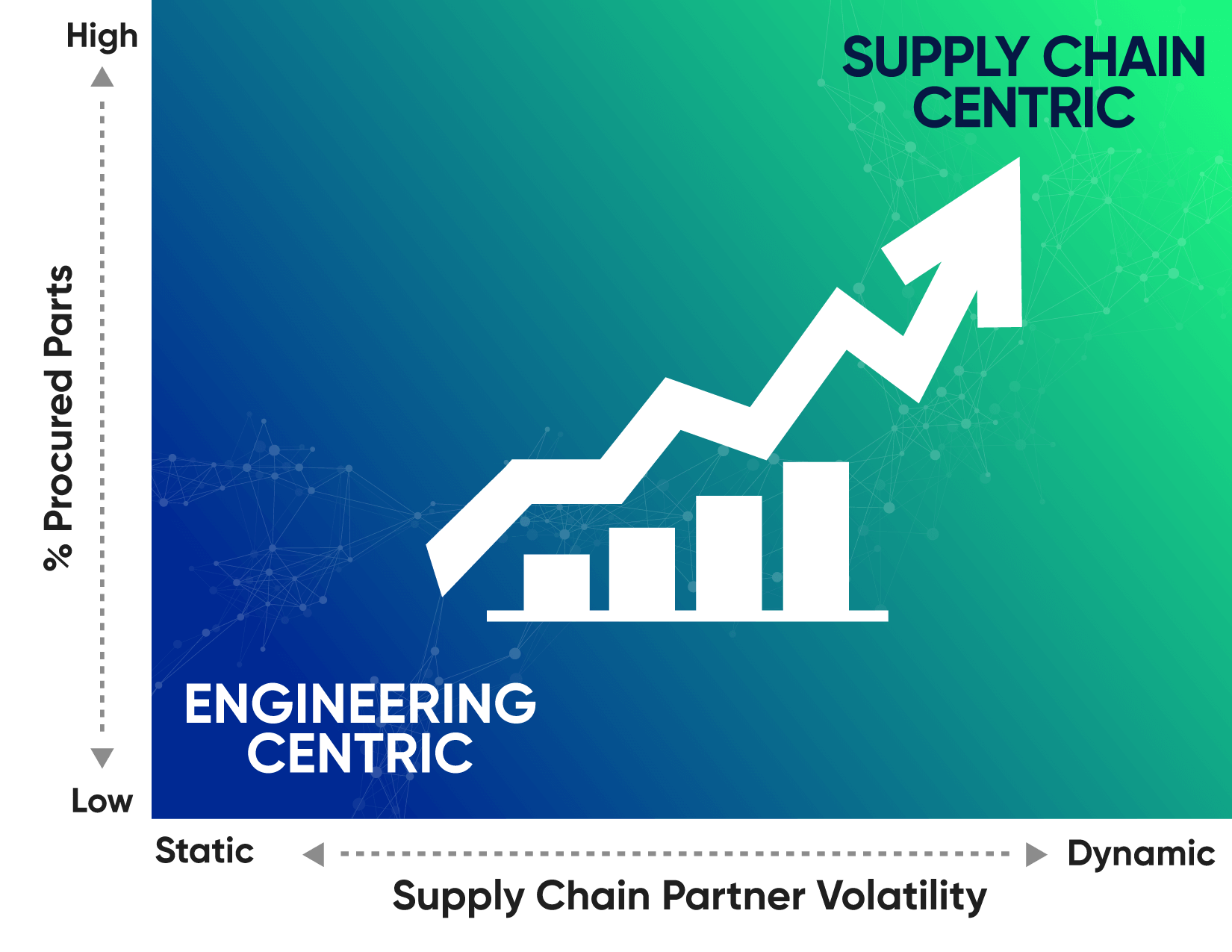 Choose the Right SaaS PLM Solution
Choose the Right SaaS PLM Solution
 Where should mold makers focus to boost business profitability?
With significant business pressures and the technical complexity of injection molding, mold making is a challenging business. While tool makers face many obstacles, those surveyed identified five top challenges that make it harder to maintain a successful business. This blog post reveals these top five challenges, and the strategies to deal with them.
Read the full guest post on the Siemens NX Design Blog.
[post_title] => How Mold Makers Can Improve Their Business
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-mold-makers-can-improve-their-business-guest-post
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-25 18:36:48
[post_modified_gmt] => 2024-01-25 23:36:48
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9130
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[19] => WP_Post Object
(
[ID] => 9332
[post_author] => 2
[post_date] => 2020-07-08 07:30:24
[post_date_gmt] => 2020-07-08 11:30:24
[post_content] =>
Where should mold makers focus to boost business profitability?
With significant business pressures and the technical complexity of injection molding, mold making is a challenging business. While tool makers face many obstacles, those surveyed identified five top challenges that make it harder to maintain a successful business. This blog post reveals these top five challenges, and the strategies to deal with them.
Read the full guest post on the Siemens NX Design Blog.
[post_title] => How Mold Makers Can Improve Their Business
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-mold-makers-can-improve-their-business-guest-post
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-25 18:36:48
[post_modified_gmt] => 2024-01-25 23:36:48
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=9130
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[19] => WP_Post Object
(
[ID] => 9332
[post_author] => 2
[post_date] => 2020-07-08 07:30:24
[post_date_gmt] => 2020-07-08 11:30:24
[post_content] =>  How can manufacturers most effectively design, develop, and launch custom-engineered products that must be engineered to customer specifications? Tech-Clarity's eBook, Bringing Custom-Engineered Products to Market, shares survey data from over 200 companies to offer insights and best practices.
Please enjoy the summary* below. Click here for the full report, thank you to our sponsor Propel (registration required).
How can manufacturers most effectively design, develop, and launch custom-engineered products that must be engineered to customer specifications? Tech-Clarity's eBook, Bringing Custom-Engineered Products to Market, shares survey data from over 200 companies to offer insights and best practices.
Please enjoy the summary* below. Click here for the full report, thank you to our sponsor Propel (registration required).
Table of Contents
- Custom-Engineered Products are Compelling
- What Does Profitable Customization Look Like?
- Developing Engineered Products is Difficult
- Benchmarking Engineered Product Performance
- Top Performers Collaborate more Readily
- Top Performers Integrate Data and Processes
- Top Performers Integrate Systems
- Conclusions and Next Steps
- About the Research
- Acknowledgments
Custom-Engineered Products are Compelling
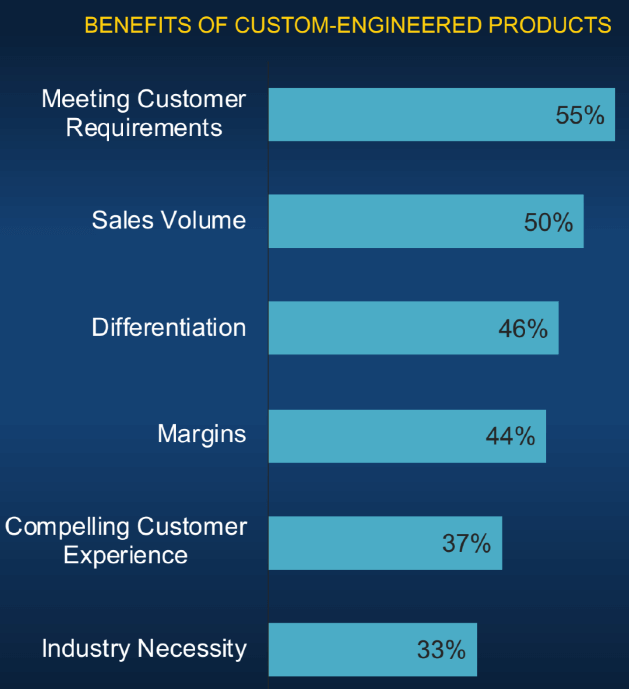 Custom-Engineering Increases the Top Line
Product customization has increased in recent years and is expected to continue to grow "significantly" for most companies over the next several years. What's driving this expansion? The benefits reported by our survey respondents help explain why.
Customization Improves the Bottom Line
Driving higher sales is no guarantee of greater profitability. If custom-engineering results in selling products too cheaply or allowing costs to get out of control, the company can still lose money. Fortunately, 44% of respondents also share the custom-engineered products help them achieve higher sales margins. Whatever the cause, higher margins help ensure that the top-line benefits of custom-engineered products fall to the bottom line.
Custom-Engineering Increases the Top Line
Product customization has increased in recent years and is expected to continue to grow "significantly" for most companies over the next several years. What's driving this expansion? The benefits reported by our survey respondents help explain why.
Customization Improves the Bottom Line
Driving higher sales is no guarantee of greater profitability. If custom-engineering results in selling products too cheaply or allowing costs to get out of control, the company can still lose money. Fortunately, 44% of respondents also share the custom-engineered products help them achieve higher sales margins. Whatever the cause, higher margins help ensure that the top-line benefits of custom-engineered products fall to the bottom line.
What Does Profitable Customization Look Like?
Custom Markets Require NPDI Speed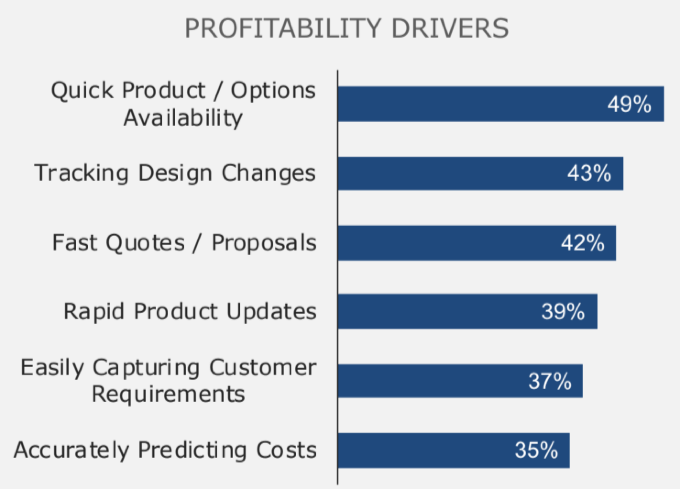 Speed without control, however, can be counterproductive. It could lead to errors or delays if companies don’t design quality into their designs upfront. These problems can have a significant impact considering that respondents say most of their engineering customization happens during customer inquiry / investigation phase and/or during quote / proposal stage – when time is at a premium.
Speed without control, however, can be counterproductive. It could lead to errors or delays if companies don’t design quality into their designs upfront. These problems can have a significant impact considering that respondents say most of their engineering customization happens during customer inquiry / investigation phase and/or during quote / proposal stage – when time is at a premium.
Developing Engineered Products is Difficult
NPDI and Engineering Challenges Impact Performance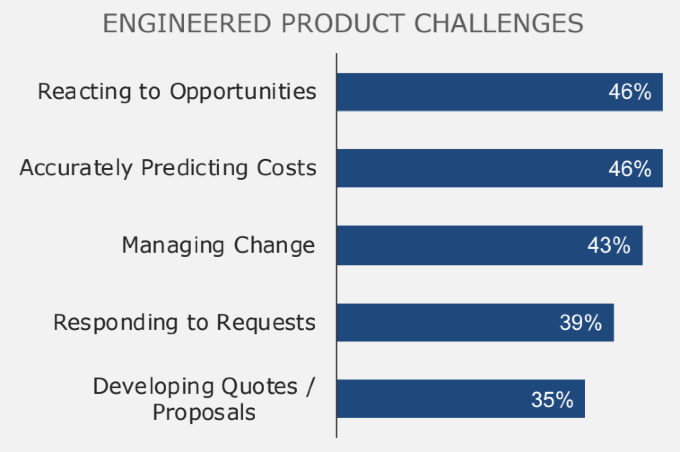 The challenges highlighted create serious consequences. Survey respondents indicate that these challenges lead to low margins, inefficiency, unhappy customers, rework/scrap, lost orders, missed market opportunities, and more. These are the negative business consequences of speed without control.
The challenges highlighted create serious consequences. Survey respondents indicate that these challenges lead to low margins, inefficiency, unhappy customers, rework/scrap, lost orders, missed market opportunities, and more. These are the negative business consequences of speed without control.
Conclusions and Next Steps
Custom-Engineered Products Deliver Benefits Offering custom-engineered products drives significant business benefits including increased top and bottom-line results. The Top Performers, the 23% with the highest performance in critical market success criteria, are even more likely to enjoy these benefits. They are also more likely to report benefits that aren’t achieved as frequently by Others. For example, about one-half of Top Performers report employee productivity as a benefit of offering custom-engineered products, which is are more than twice as frequently as Others. It’s likely that their better practices allow them not only to achieve great benefits but also to do so in a more streamlined and efficient way. Next Steps Product customization is growing. For those companies that are already taking advantage of this opportunity, the best practices identified by this research can serve as a guide for continuous improvement. For others that are embarking on adopting a custom-engineered product strategy, the practices of the Top Performers can be used to gauge your company’s readiness to deliver the speed and control required to succeed. *This summary is an abbreviated version of the research and does not contain the full content. For the full research, please visit our sponsor Propel (registration required). If you have difficulty obtaining a copy of the research, please contact us. [post_title] => Bringing Custom-Engineered Products to Market (survey results) [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => custom-engineered-products [to_ping] => [pinged] => [post_modified] => 2025-01-31 11:40:12 [post_modified_gmt] => 2025-01-31 16:40:12 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=9332 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) ) [post_count] => 20 [current_post] => -1 [before_loop] => 1 [in_the_loop] => [post] => WP_Post Object ( [ID] => 9978 [post_author] => 2574 [post_date] => 2020-11-16 11:18:58 [post_date_gmt] => 2020-11-16 16:18:58 [post_content] => How can manufacturers maximize value from their digital transformation efforts in 2021? In these uncertain times, it matters. There are some basics to leveraging Industry 4.0 trends. Success may be closer to your grasp than you realize.
This live webinar has passed, but please click here to watch it free, on-demand. Find out where trends are taking us and hear examples of how other manufacturers are already gaining benefits:
How can manufacturers maximize value from their digital transformation efforts in 2021? In these uncertain times, it matters. There are some basics to leveraging Industry 4.0 trends. Success may be closer to your grasp than you realize.
This live webinar has passed, but please click here to watch it free, on-demand. Find out where trends are taking us and hear examples of how other manufacturers are already gaining benefits:
- Agility and flexibility: Can you handle uncertainty and respond as the market changes? This is at the heart of Industry 4.0. It's also crucial to navigating the effects of COVID-19.
- Build from a strong foundation: Will your new technology projects fail? Discover why they are doomed unless you have strong enterprise systems as underpinning.
- Smooth data flows: Do your people always have a clear source of the truth? Why master data management is essential to speed and agility of response.
- New business models: Can your company stay profitable and competitive in 2021? How manufacturers can move toward added-value services, improving sustainability, and new channels to market.
All Results for "All"
What’s the Cost of Poor Collaboration?
How much does poor collaboration cost your company? Tech-Clarity’s eBook, What’s the Cost of Poor Collaboration? examines this topic for product development, sharing survey results from 155 manufacturers. Collaboration impacts every part of product development, and products cannot be developed or brought to market without it. While collaboration can be abstract and hard to measure,…
Sharing Ideas on Cloud Adoption with Siemens (podcast)
How are companies adopting design and engineering solutions on the cloud? Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives and examples. You can also see related video interviews including: Engineering in the Cloud Conversation and Designing on the Cloud Discussion with Paul Brown, Digital Transformation Progress with Bob…
The Challenge of Manufacturing Data Management (article)
What happens when automation and plant IT teams get past their differences and work together effectively? A chance for the company to outperform the competition and make progress on Industry 4.0 projects, based on more coherent manufacturing data management. In our recent research, Top Performers were more likely to have tightly connected IT and automation…
Engineering in the Cloud Conversation with Siemens (podcast)
How is the availability of cloud applications for engineering changing the way people work? Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives. You can also see related video interviews including: Designing on the Cloud Discussion with Paul Brown, Digital Transformation Progress with Bob Jones, Cloud Progress…
Cloud Readiness (infographic)
What should your company consider when transitioning to the cloud for innovation and engineering? Our new infographic identifies key considerations ranging from strategy through adoption. See the infographics for some important factors related to strategy, functionality, governance, existing solutions, and your transition. You can also learn more about cloud solutions from Siemens Digital Industries Software, our…
Meeting the Manufacturing Data Management Challenge (webcast)
What do manufacturers need to do to succeed and get results from their Industry 4.0 efforts? In this webcast, Julie Fraser revealed a few significant findings of research of over 300 companies on the topic of manufacturing data management. It’s a multi-faceted challenge in which people, processes, and technology all matter. This webcast explains the…
The Manufacturing Data Challenge (survey results)
How do companies make progress toward Industry 4.0? Based on our research, those who invest in people, processes, and technology for manufacturing data management have made more strides in Industry 4.0. Manufacturing Data Management: Lessons from Top Performers explores the many challenges of bringing together all the data production facilities need. Please enjoy the summary*…
Designing on the Cloud Discussion with Siemens (podcast)
How is the cloud playing a role in engineers’ design processes? Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software Senior Marketing Director Paul Brown to share perspectives and examples. You can also see related video interviews including: Digital Transformation Progress with Bob Jones, Cloud Progress Report with Bill Boswell, and Siemens Digital Transformation Progress…
How does CAD on mobile devices help?
Are mobile devices ready for CAD? Is CAD ready for mobile devices? When should you consider use cases for CAD on a tablet? CAD tools have evolved significantly over the last several decades, which has coincided with several platform shifts. With the advancements in tablets combined with evolutions in CAD, we may be at the…
Digital Transformation at Siemens’ Customers (podcast)
How has digital transformation progressed for Siemens’ customers and how are they leveraging Siemens’ cloud technologies to fuel their digital initiatives? How have Siemens’ customers with a digital mindset faired differently than others during the COVID-19 disruption? Tech-Clarity’s Jim Brown got together (virtually) with Siemens Digital Industries Software EVP Bob Jones to share perspectives and…
Business Sustainability and Survival 2020 (survey results)
How are companies adjusting their strategies to survive COVID-19 disruption without losing sight of long-term business sustainability? Read our survey of over 190 companies to find out how they are prioritizing limited resources to maintain focus on success factors including: Addressing changing business models Adopting new technology Protecting the environment Please enjoy the summary* below….
Buyer’s Guide for Electrical Design
What software capabilities will help you improve how you integrate mechanical, electronics, and electrical designs? Tech-Clarity’s buyer’s guide, How to Select the Ideal Solution for Today’s Smart Products: Buyer’s Guide for Electrical Design, explores this question. As companies strive to make modern products smarter, innovative and more affordable, the integration of mechanical, electrical and electronic…
Succeeding with Industry 4.0: Understanding the Whole Elephant (article)
What does effective storytelling have to do with succeeding with Industry 4.0? Plenty. With the right narrative by level and function, the whole company can engage with Industry 4.0 strategy. At that point, everyone, whether top-level management or shop-floor workers, can buy-in. Once they do, they can participate effectively in setting the standards required to…
Engineering Skills Gap in the Age of Digitalization
How can schools best prepare today’s STEM students to close the engineering skills gap so that new graduates are ready for the real world? Tech-Clarity’s research report, “How Academia Can Close the Engineering Skills Gap in the Age of Digitalization” explores this question. This research shares a global perspective on what colleges and universities are…
Supply Chain-Centric Cloud PLM (eBook)
What should supply chain-centric innovators consider when looking for PLM? How has the PLM landscape changed over the last several years, and how does SaaS fit into that picture? Please enjoy the summary* below. For the full research, please visit our sponsor, Upchain (registration required). Table of Contents The State of Cloud PLM Why Cloud SaaS is…
Digital Transformation Progress with Siemens SVP Brenda Discher (video)
What progress has Siemens made in helping themselves, and their customers, leverage the cloud for digital transformation? In this episode of Tech-Clarity TV, Tech-Clarity’s Jim Brown and Siemens SVP of Business Strategy & Marketing Brenda Discher sit down virtually to have a great discussion despite the challenges of a remote interview compounded by poor bandwidth…
Cloud Progress Report with Siemens VP Bill Boswell (video)
How is the industrial software cloud transition coming along? This episode of Tech-Clarity TV focuses on the cloud progress report for industrial software including the cloud priorities and value in the new normal. Tech-Clarity’s Jim Brown and Siemens’ VP of Cloud Marketing Bill Boswell discuss the unique and strategic value of industrial software and give a…
How Mold Makers Can Improve Their Business
Where should mold makers focus to boost business profitability? With significant business pressures and the technical complexity of injection molding, mold making is a challenging business. While tool makers face many obstacles, those surveyed identified five top challenges that make it harder to maintain a successful business. This blog post reveals these top five challenges,…
Bringing Custom-Engineered Products to Market (survey results)
How can manufacturers most effectively design, develop, and launch custom-engineered products that must be engineered to customer specifications? Tech-Clarity’s eBook, Bringing Custom-Engineered Products to Market, shares survey data from over 200 companies to offer insights and best practices. Please enjoy the summary* below. Click here for the full report, thank you to our sponsor Propel (registration…