How do you bridge the gulf between product engineering and manufacturing? In the product lifecycle, manufacturing sits at the center. Rapid product and process improvement, lower cost of quality, and business innovation rest on engineering and manufacturing working together effectively. Yet often they don’t or can’t. Tech-Clarity research shows that MES and PLM are the…
- Cloud PLM Adoption Questions Shift
- Increase the Value of PLM with SaaS
- Buyer's Considerations
- Review Deployment Options
- Considerations for Adoption
- Evaluate Pricing Options
- Product Development Needs
- Operational Considerations
- Choosing the Right PLM
- Choose the Right Partner
- Special Considerations
- Conclusions and Recommendations
- Acknowledgments
PLM is the Digital Backbone for Manufacturing
Considerations for Digital Transformation with PLM The pace of business is accelerating and companies must digitally transform to compete. Our research finds that PLM is critical to manufacturers’ digital transformations and initiatives including the digital twin and the digital thread. Today’s PLM system must serve as the product backbone of the digital manufacturing enterprise. But too many companies are stuck on old, outdated versions of their PLM system or need to move to the cloud to support their goals. Cloud SaaS can provide the modern, full featured PLM capabilities companies need while increasing agility, speed, collaboration, performance, availability, and security and simultaneously reducing cost, time to value, risk, and IT overhead. Not all “cloud” solutions, however, support digital transformation in the same way. Manufacturers should closely examine their requirements and be careful not to trade off essential PLM capabilities to meet those requirements.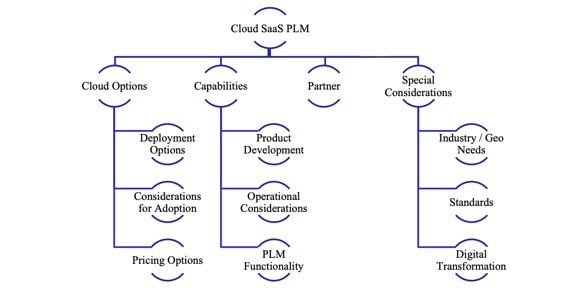
Cloud PLM Adoption Questions Shift
The Shift from “If” Cloud PLM to “When and How” As recently as our 2019 buyer’s guide we observed that more companies were starting to ask “why not the cloud?” instead of “why consider cloud?” Although some companies and industries may still have obstacles that prevent them from moving to the cloud, cloud PLM solutions are quickly becoming the preferred approach. Our research shows that over one-half of manufacturers are considering cloud and about one-quarter already leverage the cloud to support product innovation and manufacturing1. Now the questions are “when and how?” to adopt Cloud PLM. Navigating the Options Choosing to move to the cloud is just the first step. There are still important decisions to make. The deployment choice impacts important factors including cost, security, resource requirements, performance, availability, upgradability, risk, and time to benefit. While it’s clear that the software industry is moving to a cloud SaaS model overall, not all cloud PLM solutions are following that model. Manufacturers need to go deeper into the deployment model than just “cloud.” This guide helps companies navigate the options and choose the best-suited cloud PLM solution for their business. Still a Solution First Approach Although the priority for cloud solutions has increased, our surveys still show that the majority of companies put a higher priority on PLM capabilities than cloud deployment. They recognize that they don’t just need a cloud PLM system, they need a fully-featured PLM system on the cloud. Over ½ of companies stated that they were willing to give up “very little” or “no” functionality as a tradeoff for the IT benefits of the cloud.2 Companies are still not willing to shortchange functionality in this crucial area, effectively taking a “solution first” approach as opposed to a “cloud first.” Therefore, it’s important to evaluate the functional capabilities of a PLM system to ensure it will deliver the significant top- and bottom-line benefits they need from PLM.Conclusions and Recommendations
 Cloud SaaS Offers Compelling Benefits
Cloud SaaS helps manufacturers achieve and extend the significant business value of PLM faster, with less risk, and lower total cost of ownership. It offers new opportunities to enhance global reach, secure design sharing, and collaboration. At the same time, it offers compelling operational benefits such as improved performance, security, access to new functionality, and scalability. But there are important things to consider when selecting a cloud PLM system, ranging from deployment options to considerations for certain industries and geographies.
Recommendations for Cloud PLM Selection
To help companies research and analyze potential solutions based on company needs, including needs that help deliver benefits well into the future, Tech-Clarity offers the following recommendations:
Cloud SaaS Offers Compelling Benefits
Cloud SaaS helps manufacturers achieve and extend the significant business value of PLM faster, with less risk, and lower total cost of ownership. It offers new opportunities to enhance global reach, secure design sharing, and collaboration. At the same time, it offers compelling operational benefits such as improved performance, security, access to new functionality, and scalability. But there are important things to consider when selecting a cloud PLM system, ranging from deployment options to considerations for certain industries and geographies.
Recommendations for Cloud PLM Selection
To help companies research and analyze potential solutions based on company needs, including needs that help deliver benefits well into the future, Tech-Clarity offers the following recommendations:
- Evaluate functional solution capabilities to ensure that the PLM solution provides the rich capabilities required to support your business.
- Recognize that there are significant differences in “cloud” PLM offerings.
- Evaluate and select the optimal deployment and pricing models that give your business the most benefit considering cost, risk, and time to achieve value.
- Consider how the deployment approach will impact future financial and operational value during updates, upgrades, and extensions
- Consider the strategic value of cloud solutions for global deployments to support global design environments, remote workers, and secure supply chain collaboration.
- Make sure to consider the future, including the transition to the digital enterprise. We believe that manufacturers that don’t digitally transform will be at a competitive disadvantage.
- Narrow down solutions based on these high-level criteria to create a smaller list of solutions to evaluate.
- Recognize that any solution selection process will require tradeoffs and understand which types of requirements are the most important to your company’s success and profitability.
 Companies are making a significant shift to the cloud, how is that impacting PLM? What do manufacturers need to be aware of related to features, customization, and upgrades as they make the transition? Our survey results include insights from over one hundred manufacturers to find out.
Please enjoy the summary below.* For the full report, please visit our sponsor Aras (registration required).
For related content, watch the interactive webinar replay where Jim Brown shares in-depth insights from his conversations with customers.
Companies are making a significant shift to the cloud, how is that impacting PLM? What do manufacturers need to be aware of related to features, customization, and upgrades as they make the transition? Our survey results include insights from over one hundred manufacturers to find out.
Please enjoy the summary below.* For the full report, please visit our sponsor Aras (registration required).
For related content, watch the interactive webinar replay where Jim Brown shares in-depth insights from his conversations with customers.
Table of Contents
- PLM is Moving to the Cloud
- Cloud Transformation Patience is Over
- Current State of PLM Boosts Cloud Opportunity
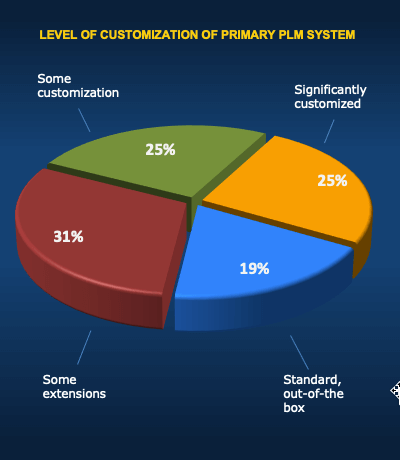
- Take an Objective Look at Customization
- Plan for the Reality of Customization
- Understand Upgrade Processes
- Ensure PLM Delivers Business Value
- Conclusions
- About the Research
- Acknowledgments
Cloud PLM Adoption Accelerating
Develop Cloud Transition Strategy Companies are making a significant shift to cloud software. To get a clearer picture of the manufacturing industry’s current state and future plans for cloud PLM, Tech-Clarity surveyed over one hundred larger manufacturers with over 500 employees. Survey results show that cloud PLM adoption is accelerating and becoming the norm. Understand Implications Manufacturers favor cloud solutions and the majority now use, or plan to use, cloud PLM. Although most companies are familiar with the benefits of the cloud, not all companies understand the implications of cloud deployment model and architecture choices. In particular, this survey examines several important considerations for ensuring they drive business value from their PLM implementation, including the impacts on:- Features needed to reach business goals
- Customization to achieve business value and ensure user adoption
- Upgrade timing to access innovation while remaining compliant
PLM is Moving to the Cloud
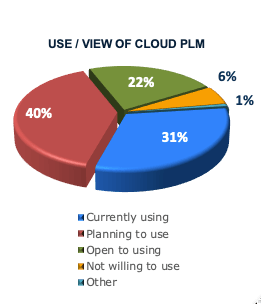 Manufacturers Now Favor the Cloud
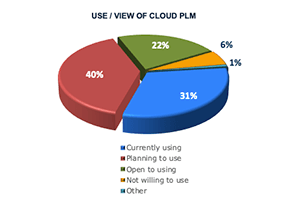
The shift to cloud software is a relatively recent, fundamental shift in IT architecture. Only 17% of companies participating in our 2018 study1 had a software strategy that called for only using the cloud, using the cloud unless no capable solution was available, or preferring the cloud. Instead, over one-third would choose the most capable solution and about one-quarter did not consider or allow the cloud.
The 2022 survey reflects a dramatically different climate. Now, about three-quarters of companies favor, prefer, or mandate the cloud for their new software selections. In fact, only 4% of responding companies say they do not consider or allow the cloud.
PLM Cloud Transformation has Accelerated
Those results detail companies’ overall cloud strategy, but how does that relate to PLM? PLM adoption has traditionally lagged behind other enterprise software solutions. While some attributed lower adoption to security concerns, we believe it was because companies were forced to choose between cloud solutions or fully-featured PLM. Either way, it’s clear that the PLM transformation is now picking up pace. Almost three-quarters of surveyed companies are either using cloud PLM or to planning to do so. Very few, on the other hand, are unwilling to use the cloud. Our experience shows that this is likely due to regulatory or customer mandates.
Manufacturers Now Favor the Cloud
The shift to cloud software is a relatively recent, fundamental shift in IT architecture. Only 17% of companies participating in our 2018 study1 had a software strategy that called for only using the cloud, using the cloud unless no capable solution was available, or preferring the cloud. Instead, over one-third would choose the most capable solution and about one-quarter did not consider or allow the cloud.
The 2022 survey reflects a dramatically different climate. Now, about three-quarters of companies favor, prefer, or mandate the cloud for their new software selections. In fact, only 4% of responding companies say they do not consider or allow the cloud.
PLM Cloud Transformation has Accelerated
Those results detail companies’ overall cloud strategy, but how does that relate to PLM? PLM adoption has traditionally lagged behind other enterprise software solutions. While some attributed lower adoption to security concerns, we believe it was because companies were forced to choose between cloud solutions or fully-featured PLM. Either way, it’s clear that the PLM transformation is now picking up pace. Almost three-quarters of surveyed companies are either using cloud PLM or to planning to do so. Very few, on the other hand, are unwilling to use the cloud. Our experience shows that this is likely due to regulatory or customer mandates.
Conclusions
 The Transition is Underway
A fundamental cloud PLM transition is underway and has accelerated. Despite a slower start than some other applications like ERP or CRM, manufacturers are rapidly pursuing cloud PLM solutions. Cloud PLM offers significant benefits, and companies appear to have transitioned from deciding “if” to implement a cloud solution for PLM and now are discussing “when” and “how.”
Cloud Transformation Offers Additional Improvement Opportunities
In addition to cloud benefits, the large number of companies planning to replace their PLM system with a cloud offering can benefit from a “reset” if their PLM system does not move to the cloud quickly enough. This reset may be valuable because the common state of PLM hampers progress and value due to:
The Transition is Underway
A fundamental cloud PLM transition is underway and has accelerated. Despite a slower start than some other applications like ERP or CRM, manufacturers are rapidly pursuing cloud PLM solutions. Cloud PLM offers significant benefits, and companies appear to have transitioned from deciding “if” to implement a cloud solution for PLM and now are discussing “when” and “how.”
Cloud Transformation Offers Additional Improvement Opportunities
In addition to cloud benefits, the large number of companies planning to replace their PLM system with a cloud offering can benefit from a “reset” if their PLM system does not move to the cloud quickly enough. This reset may be valuable because the common state of PLM hampers progress and value due to:
- Outdated PLM systems that have not been recently upgraded, resulting in missed opportunity to take advantage of functional and technical enhancement opportunities. Our experience shows this is frequently due to dead-end customization approaches that make upgrades extremely challenging.
- Multiple PLM systems, leading to high cost and operational disadvantages that limit PLM value.
- The need and ability to customize PLM
- The requirements and limitations related to controlling PLM upgrade timing
- The ability to deliver the rich PLM capabilities they need to deliver value
 [post_title] => Improving Continuous Improvement
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => improving-continuous-improvement-infographic
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-02 13:22:22
[post_modified_gmt] => 2024-01-02 18:22:22
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11693
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[4] => WP_Post Object
(
[ID] => 11660
[post_author] => 2
[post_date] => 2022-07-25 09:30:08
[post_date_gmt] => 2022-07-25 13:30:08
[post_content] =>
[post_title] => Improving Continuous Improvement
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => improving-continuous-improvement-infographic
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-02 13:22:22
[post_modified_gmt] => 2024-01-02 18:22:22
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11693
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[4] => WP_Post Object
(
[ID] => 11660
[post_author] => 2
[post_date] => 2022-07-25 09:30:08
[post_date_gmt] => 2022-07-25 13:30:08
[post_content] =>  What does the future look like for PLM in the CPG industry? Tech-Clarity led an interactive discussion with Pepsico and Kalypso PLM leaders in a Kalypso-hosted webinar. It was an interactive discussion with PLM industry leaders from a variety of roles to discuss the findings of our recent research on the Future of PLM in CPG.
The panel brought together over six decades of PLM experience from industry, trusted advisors, and research perspectives. The panel, hosted by Kalypso's Consumer Industry Marketing Manager Hadley Bauer, consisted of:
What does the future look like for PLM in the CPG industry? Tech-Clarity led an interactive discussion with Pepsico and Kalypso PLM leaders in a Kalypso-hosted webinar. It was an interactive discussion with PLM industry leaders from a variety of roles to discuss the findings of our recent research on the Future of PLM in CPG.
The panel brought together over six decades of PLM experience from industry, trusted advisors, and research perspectives. The panel, hosted by Kalypso's Consumer Industry Marketing Manager Hadley Bauer, consisted of:
- Susan Hamel, PepsiCo's Senior Director of Global R&D, shared her experience in advancing the PLM agenda over the last 20 years
- Colin Speakman, Kalypso's Consumer Goods Global Director, offered his experience, knowledge, and strong relationships with CPG companies
- Jim Brown, Tech-Clarity's President of Product Innovation and Digital Transformation Research
 [post_title] => Future of CPG PLM with Kalypso and Pepsico
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cpg-plm-blog
[to_ping] =>
[pinged] =>
[post_modified] => 2023-12-15 16:35:14
[post_modified_gmt] => 2023-12-15 21:35:14
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11660
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[5] => WP_Post Object
(
[ID] => 11650
[post_author] => 2574
[post_date] => 2022-07-19 10:37:40
[post_date_gmt] => 2022-07-19 14:37:40
[post_content] =>
[post_title] => Future of CPG PLM with Kalypso and Pepsico
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cpg-plm-blog
[to_ping] =>
[pinged] =>
[post_modified] => 2023-12-15 16:35:14
[post_modified_gmt] => 2023-12-15 21:35:14
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11660
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[5] => WP_Post Object
(
[ID] => 11650
[post_author] => 2574
[post_date] => 2022-07-19 10:37:40
[post_date_gmt] => 2022-07-19 14:37:40
[post_content] =>  How can strategic sourcing professionals lower risk and cost in these uncertain times? This new paper by Procurement Leaders points to the potential of AI in a marketplace, and quotes Tech-Clarity’s Julie Fraser.
Please enjoy the summary* below. For the full research, please visit Procurement Leaders (registration required).
Materials price increases and supplier risk have not abated since the start of the COVID-19 pandemic. Traditional procurement automation systems do not address those issues effectively. Thus, companies must find new ways to combat what appears to be a permanent situation.
“Flipping the pyramid” to use mostly external data and combine internal data with it may hold the key. Marketplaces are once again taking the stage, and with advanced analytics behind them, the benefits are significant. This paper has a concrete example from Bose.
Electronics is an example of an industry where many suppliers and parts are used by a multitude of companies, and available both direct from the manufacturer and from distributors. This makes it ideal for such a marketplace approach.
Fraser points out that near real-time data about supply and logistics from outside still needs a way to get into context with internal data for analytics to perform. If a platform allows that, it can truly push out insights that lead to decisions for how to improve costs and reduce risk.
Read the report to learn more. Thanks, Malcolm Wheatley for the opportunity to share our point of view along with many luminaries.
For related research with Julie Fraser, please read Procurement automation: advancing to the future.
[post_title] => Strategic Sourcing: Leverage AI to Mitigate Risk and Cut Costs (quote spotlight)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => sourcing-risk-and-cost-paper
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:58
[post_modified_gmt] => 2022-11-15 03:25:58
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11650
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[6] => WP_Post Object
(
[ID] => 17732
[post_author] => 2574
[post_date] => 2022-07-16 09:47:24
[post_date_gmt] => 2022-07-16 13:47:24
[post_content] => What can manufacturers do to gain full business value from IIoT investments? Julie Fraser shares three tips to create a firm foundation for success on The Peggy Smedley Show podcast.
Hear four examples of how new technology infrastructure and architecture can support bridging the IT/OT divide. Plus a focus on process improvement and people. All of these can deliver strong IIoT business value - if you stay focused on them. Listen to this in this episode to get some ideas for what you might do to better ensure IIoT projects deliver business benefits.
How can strategic sourcing professionals lower risk and cost in these uncertain times? This new paper by Procurement Leaders points to the potential of AI in a marketplace, and quotes Tech-Clarity’s Julie Fraser.
Please enjoy the summary* below. For the full research, please visit Procurement Leaders (registration required).
Materials price increases and supplier risk have not abated since the start of the COVID-19 pandemic. Traditional procurement automation systems do not address those issues effectively. Thus, companies must find new ways to combat what appears to be a permanent situation.
“Flipping the pyramid” to use mostly external data and combine internal data with it may hold the key. Marketplaces are once again taking the stage, and with advanced analytics behind them, the benefits are significant. This paper has a concrete example from Bose.
Electronics is an example of an industry where many suppliers and parts are used by a multitude of companies, and available both direct from the manufacturer and from distributors. This makes it ideal for such a marketplace approach.
Fraser points out that near real-time data about supply and logistics from outside still needs a way to get into context with internal data for analytics to perform. If a platform allows that, it can truly push out insights that lead to decisions for how to improve costs and reduce risk.
Read the report to learn more. Thanks, Malcolm Wheatley for the opportunity to share our point of view along with many luminaries.
For related research with Julie Fraser, please read Procurement automation: advancing to the future.
[post_title] => Strategic Sourcing: Leverage AI to Mitigate Risk and Cut Costs (quote spotlight)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => sourcing-risk-and-cost-paper
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:58
[post_modified_gmt] => 2022-11-15 03:25:58
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11650
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[6] => WP_Post Object
(
[ID] => 17732
[post_author] => 2574
[post_date] => 2022-07-16 09:47:24
[post_date_gmt] => 2022-07-16 13:47:24
[post_content] => What can manufacturers do to gain full business value from IIoT investments? Julie Fraser shares three tips to create a firm foundation for success on The Peggy Smedley Show podcast.
Hear four examples of how new technology infrastructure and architecture can support bridging the IT/OT divide. Plus a focus on process improvement and people. All of these can deliver strong IIoT business value - if you stay focused on them. Listen to this in this episode to get some ideas for what you might do to better ensure IIoT projects deliver business benefits.

[post_title] => Three Tips to Ensure IIoT Delivers Business Value [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => iiot-business-value [to_ping] => [pinged] => [post_modified] => 2024-01-02 13:31:10 [post_modified_gmt] => 2024-01-02 18:31:10 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=17732 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [7] => WP_Post Object ( [ID] => 11633 [post_author] => 2572 [post_date] => 2022-07-13 09:00:13 [post_date_gmt] => 2022-07-13 13:00:13 [post_content] =>
 What can you expect for PLM ROI? How will it help your business?
Engineers waste a lot of valuable time on non-value-added work. However, our research finds that Product Lifecycle Management (PLM) can help. It provides business value by empowering engineers to focus more of their valuable time on innovation, design, and engineering. Still, how can it help YOUR company?
Tech-Clarity's 5-minute online assessment leverages our research to help you assess and predict the business value of PLM at your company so that you will have a better understanding of PLM's Return on Investment (ROI).
What can you expect for PLM ROI? How will it help your business?
Engineers waste a lot of valuable time on non-value-added work. However, our research finds that Product Lifecycle Management (PLM) can help. It provides business value by empowering engineers to focus more of their valuable time on innovation, design, and engineering. Still, how can it help YOUR company?
Tech-Clarity's 5-minute online assessment leverages our research to help you assess and predict the business value of PLM at your company so that you will have a better understanding of PLM's Return on Investment (ROI).
The Online Assessment
This online assessment asks a few questions to understand the goals of your business, your development environment, your top challenge, and the complexity of your products. It then calculates the expected time and cost savings, plus increased revenue opportunities, based on the experiences of companies that develop similarly complex products. In addition to the calculated values, the assessment produces a customized report explaining how PLM can help your company meet its top goals and solve your biggest challenge. Take the online assessment here. You will also have the option to save, print, and share the results. Learn more about Teamcenter PLM from our sponsor, Siemens. For related research, you may be interested in our eBook, The Business Value of Reducing Engineering Time Wasters. [post_title] => PLM ROI Payback
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => plm-roi-calculator-assessment
[to_ping] =>
[pinged] =>
[post_modified] => 2023-12-15 13:17:06
[post_modified_gmt] => 2023-12-15 18:17:06
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11633
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[8] => WP_Post Object
(
[ID] => 11616
[post_author] => 2572
[post_date] => 2022-07-12 09:00:26
[post_date_gmt] => 2022-07-12 13:00:26
[post_content] =>
[post_title] => PLM ROI Payback
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => plm-roi-calculator-assessment
[to_ping] =>
[pinged] =>
[post_modified] => 2023-12-15 13:17:06
[post_modified_gmt] => 2023-12-15 18:17:06
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11633
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[8] => WP_Post Object
(
[ID] => 11616
[post_author] => 2572
[post_date] => 2022-07-12 09:00:26
[post_date_gmt] => 2022-07-12 13:00:26
[post_content] =>  What should you consider to ensure your MBE strategy is successful?
Manufacturers adopting a Model-Based Enterprise (MBE) strategy report improved traceability and agility, faster delivery, greater efficiency, and lower costs. What should you consider to realize similar benefits? An MBE is an organization that uses digital models to support commissioning, operating, servicing, and decommissioning a product, thereby eliminating many of the process and coordination challenges associated with managing products with paper-based, manual processes throughout their lifecycle.
Adopting a Model-Based Enterprise (MBE) Strategy? What You Should Know, a research report based on a survey of 250 discrete manufacturers, establishes a "state of the market" for MBE adoption. In addition, it identifies best practices and considerations to help you progress to higher maturity levels during your MBE adoption journey. This understanding should help manufacturers better plan and prepare for the journey by leveraging people, process, and technology to maximize return on investment as early as possible.
Please enjoy the summary below.* For the full report, please visit our sponsor iBASEt (registration required).
What should you consider to ensure your MBE strategy is successful?
Manufacturers adopting a Model-Based Enterprise (MBE) strategy report improved traceability and agility, faster delivery, greater efficiency, and lower costs. What should you consider to realize similar benefits? An MBE is an organization that uses digital models to support commissioning, operating, servicing, and decommissioning a product, thereby eliminating many of the process and coordination challenges associated with managing products with paper-based, manual processes throughout their lifecycle.
Adopting a Model-Based Enterprise (MBE) Strategy? What You Should Know, a research report based on a survey of 250 discrete manufacturers, establishes a "state of the market" for MBE adoption. In addition, it identifies best practices and considerations to help you progress to higher maturity levels during your MBE adoption journey. This understanding should help manufacturers better plan and prepare for the journey by leveraging people, process, and technology to maximize return on investment as early as possible.
Please enjoy the summary below.* For the full report, please visit our sponsor iBASEt (registration required).
Table of Contents
- Executive Summary
- MBE Definition
- Adopting MBE
- Top Performers
- Maturity Index
- MBE Impact
- Adoption Challenges
- Recommendations
- About the Research
- Acknowledgments
The MBE Vision
Realizing the Vision The ultimate vision for MBE is for every function to leverage the product model across the entire product lifecycle (see graphic). However, this vision will take time to realize. By planning for it now, your company can start experiencing some of the early benefits, but it can be tricky figuring out how to get started. This eBook provides guidance to help you begin, proceed along the journey, and anticipate potential challenges that could inhibit progress.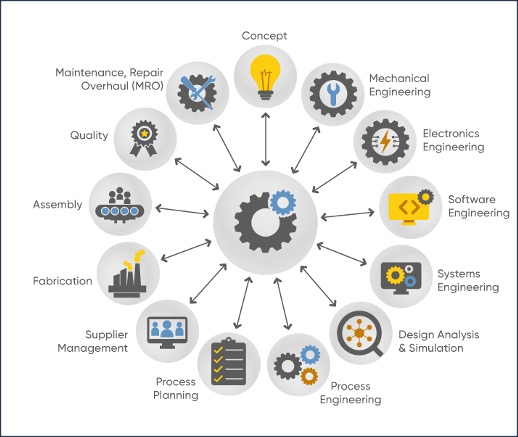
Adoption Conclusions
Challenges Evolve As companies start their journey, they run into many process challenges. However, as they work through it, process is less likely to be a top challenge. On the other hand, technology is less of an adoption hurdle early on. However, as the journey progresses and companies automate more, technology plays a more prominent role and it becomes a bigger challenge. Overall, the people and culture challenges are the biggest adoption hurdle, regardless of where companies are in the journey. Success Considerations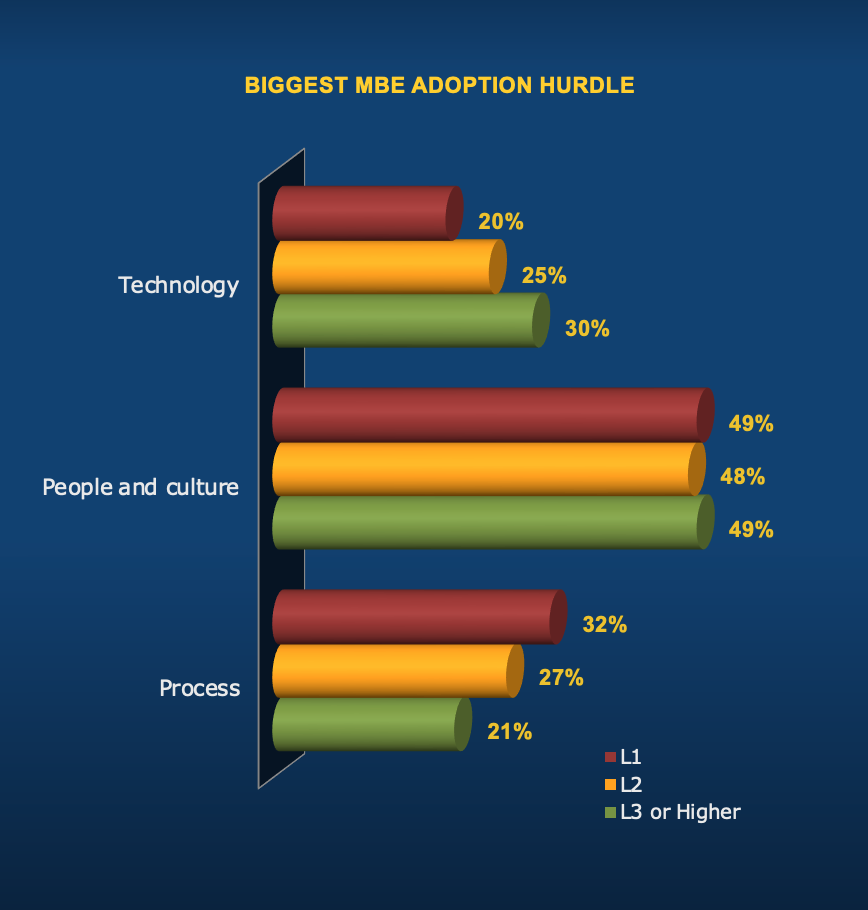 Do not let technology concerns hold you back as you start the MBE journey. MBE offers benefits early on, while technology does not become a bigger issue until later.
Upfront, think more about process. This is an opportunity to modernize and rethink how processes should work without the limitations of current approaches. Today's environment and products have evolved significantly, so processes developed decades ago will likely hold you back.
Do not overlook people and culture changes. Executive support is critical, and training needs must be addressed regularly throughout the journey. Employees need to understand the value of the new approach, why it is better for them, and how it will help them do an even better job, in less time, with higher quality.
Vendors who have a strong vision for MBE can be a great asset. Those involved in standards organizations such as NIST will likely support the latest standards. Look for software solutions that will leverage semantic PMI to automate work.
Do not let technology concerns hold you back as you start the MBE journey. MBE offers benefits early on, while technology does not become a bigger issue until later.
Upfront, think more about process. This is an opportunity to modernize and rethink how processes should work without the limitations of current approaches. Today's environment and products have evolved significantly, so processes developed decades ago will likely hold you back.
Do not overlook people and culture changes. Executive support is critical, and training needs must be addressed regularly throughout the journey. Employees need to understand the value of the new approach, why it is better for them, and how it will help them do an even better job, in less time, with higher quality.
Vendors who have a strong vision for MBE can be a great asset. Those involved in standards organizations such as NIST will likely support the latest standards. Look for software solutions that will leverage semantic PMI to automate work.
Recommendations
Recommendations and Next Steps Based on industry experience and research for this report, Tech-Clarity offers the following recommendations to adopt an MBE strategy:- Use MBE as an opportunity to rethink your business operations to enable greater efficiency, lower cost, and improve quality while better serving your customers.
- Consider using the MBE Maturity Index as a framework to guide your adoption journey.
- Ensure executives are involved enough to provide support and leadership as needed.
- Establish new processes structured around a 3D model to help overcome ingrained 2D processes.
- Use CAD tools with more mature capabilities for model-based product definitions, MBD.
- Work with software vendors who have a vision for how Operations should leverage semantic PMI. Keep in mind, MBE spans multiple domains and includes solutions for Engineering, Manufacturing, and Quality.
- Use vendors who are involved with standards organizations so that you can benefit from the best practices developed by these independent organizations.
- Do not overlook the people challenges. Meeting training needs and ensuring all staff understand the business value and benefit to them will be critical to overcome the cultural resistance to change. This should be an ongoing consideration, especially as new technology is introduced throughout the entire journey.
 What has changed in the past 30 years of manufacturing? Listen to the Manufacturing IT Podcast episode where Julie Fraser shares her thoughts with host Daniel Langley. We wander through topics from cigars and sexism to rebranding manufacturing
and remaking companies to be more customer-centric and serious about ESG to overcome the skills shortage. You’ll hear the excitement of myriad new technologies allowing visions of data-driven operations coming to reality. We touch on research and why technology matters to businesses.
Watch the podcast on YouTube or listen on Apple Music.
[post_title] => Julie Fraser on The Manufacturing IT Podcast with Daniel Langley
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => manufacturing-it-podcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:58
[post_modified_gmt] => 2022-11-15 03:25:58
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11601
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[10] => WP_Post Object
(
[ID] => 11590
[post_author] => 2574
[post_date] => 2022-07-01 10:47:36
[post_date_gmt] => 2022-07-01 14:47:36
[post_content] =>
What has changed in the past 30 years of manufacturing? Listen to the Manufacturing IT Podcast episode where Julie Fraser shares her thoughts with host Daniel Langley. We wander through topics from cigars and sexism to rebranding manufacturing
and remaking companies to be more customer-centric and serious about ESG to overcome the skills shortage. You’ll hear the excitement of myriad new technologies allowing visions of data-driven operations coming to reality. We touch on research and why technology matters to businesses.
Watch the podcast on YouTube or listen on Apple Music.
[post_title] => Julie Fraser on The Manufacturing IT Podcast with Daniel Langley
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => manufacturing-it-podcast
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:58
[post_modified_gmt] => 2022-11-15 03:25:58
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11601
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[10] => WP_Post Object
(
[ID] => 11590
[post_author] => 2574
[post_date] => 2022-07-01 10:47:36
[post_date_gmt] => 2022-07-01 14:47:36
[post_content] =>  Has the time come to do continuous improvement (CI) on the approach to CI? We think so. There is a new era in manufacturing, so it’s time for a new era in continuous improvement programs. We interviewed manufacturers, consultants, and associations to validate the notion we explain in this white paper.
Please enjoy the summary* below. For the full research please visit our sponsor, PTC (registration required).
For related research, read Getting Beyond Percentages to Insights with OEE to learn how to accelerate improvements with your equipment.
Has the time come to do continuous improvement (CI) on the approach to CI? We think so. There is a new era in manufacturing, so it’s time for a new era in continuous improvement programs. We interviewed manufacturers, consultants, and associations to validate the notion we explain in this white paper.
Please enjoy the summary* below. For the full research please visit our sponsor, PTC (registration required).
For related research, read Getting Beyond Percentages to Insights with OEE to learn how to accelerate improvements with your equipment.
Table of Contents
- Executive Overview
- New Era in Manufacturing
- The Problem-Solving Story
- Five New-Era CI Needs
- New Era in Manufacturing
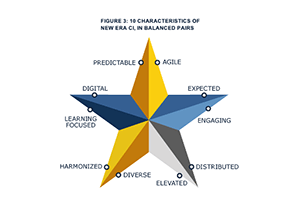
- Expected and Engaging
- Distributed and Elevated
- Diverse Yet Harmonized
- Learning-focused and Digital
- Enabling CI Sequels
- Recommendations
- References
- About the Author
Executive Overview
There’s a new Era in Manufacturing; it is time for a new era of continuous improvement (CI). This is a time of digital approaches delivering an array of new capabilities and insights that can move the business beyond unpleasant trade-offs. In this new era, CI gets enterprise-wide standardization and support and keeps the enthusiasm of local teams and employees. It delivers both local breakthroughs and views that support executives in understanding progress and prioritizing resources for the following CI projects in ways that make sense to everyone. Appropriate digitalization enables CI teams to get reliable access to all of the data they need from a wide variety of sources. Beyond that, it supports them in gaining rich insights from the data with far less effort than ever before. Goals and results are translated into a shared, visceral unit of improvement that matters to shop floor operators, supervisors, managers, and executives: time. By feeding shared understanding, this digitally-supported CI is more likely to keep generating enthusiasm and benefits even as the business shifts and changes.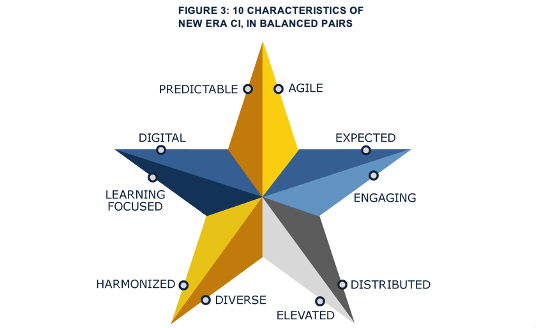
Recommendations
Based on industry experience and research for this report, Tech-Clarity offers the following recommendations:- Assess and understand how well your CI program matches your current and future needs for competitiveness and responsiveness to the unexpected
- Continue to train on CI best practices from organizations such as AME
- Make CI systematic, and begin to ingrain good practices in the culture and processes
- Engage everyone: top executives through all levels of management to associates
- Leverage industry resources such as MESA’s Analytics Guidebook to develop a strategy that harmonizes business and operational metrics
- Go beyond financial to time metrics to harmonize and prioritize projects
- Explore modern software explicitly designed to support performance improvement and measurement in manufacturing
- Use digital means to support your CI process and your organization’s ongoing success
[post_title] => A New Era of Manufacturing Continuous Improvement [post_excerpt] => [post_status] => publish [comment_status] => open [ping_status] => open [post_password] => [post_name] => continuous-improvement-whitepaper [to_ping] => [pinged] => [post_modified] => 2024-01-02 13:36:58 [post_modified_gmt] => 2024-01-02 18:36:58 [post_content_filtered] => [post_parent] => 0 [guid] => https://tech-clarity.com/?p=11590 [menu_order] => 0 [post_type] => post [post_mime_type] => [comment_count] => 0 [filter] => raw ) [11] => WP_Post Object ( [ID] => 11576 [post_author] => 2574 [post_date] => 2022-06-28 00:30:06 [post_date_gmt] => 2022-06-28 04:30:06 [post_content] =>
 Customer expectations for rapid, complete shipments are rising constantly. How can a business set supply chain strategy to meet current and future needs? Listen to this webcast from July 19, 2022 where Julie Fraser explores this with Kenny William of Parts Town and Victoria Brown of Körber Supply Chain. Both have deep experience in ensuring that warehouse management software (WMS) keeps products moving even when customer expectations change.
You will hear and learn about:
Customer expectations for rapid, complete shipments are rising constantly. How can a business set supply chain strategy to meet current and future needs? Listen to this webcast from July 19, 2022 where Julie Fraser explores this with Kenny William of Parts Town and Victoria Brown of Körber Supply Chain. Both have deep experience in ensuring that warehouse management software (WMS) keeps products moving even when customer expectations change.
You will hear and learn about:
- how to manage uncertainty
- examples of how to stay agile
- WMS software flexibility and ability to support constant change
- keeping up with process improvements
- accelerating movement through the warehouse
 Beyond implementing lab technologies, what can laboratories do to leverage them effectively? We surveyed 222 people working in corporate labs to find out.
Please enjoy the summary* below. For the full research, please visit our sponsor Dassault Systemès BIOVIA (registration required).
Beyond implementing lab technologies, what can laboratories do to leverage them effectively? We surveyed 222 people working in corporate labs to find out.
Please enjoy the summary* below. For the full research, please visit our sponsor Dassault Systemès BIOVIA (registration required).
Table of Contents
- Current Situation
- Challenges
- Technology - The Solution?
- Additional Needs
- Recommendations
- About the Research
- Acknowledgements
Introduction
Technology Pays Off, but Maximum Performance Requires More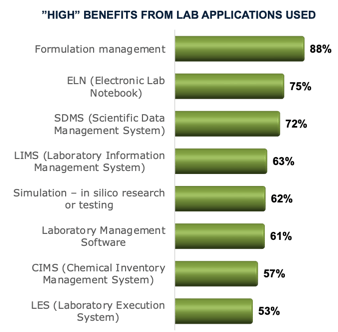 Can new technology in the laboratory help make companies more successful? Apparently. Almost universally, those who use them report they deliver significant business benefits. Yet constant changes, including changes in processes, materials, and technology, create challenges. To minimize inefficiency, most labs have worked toward good processes and practices. All of that is still not delivering total potential value.
This research of 222 respondents from labs worldwide indicates that new technologies and processes are insufficient. Very few respondents report good – let alone excellent – performance on throughput, cycle time, documentation for compliance, or analyzing data and making sound decisions. It appears that greater integration and data standardization, a data-centric mindset, and process shift are also required.
Can new technology in the laboratory help make companies more successful? Apparently. Almost universally, those who use them report they deliver significant business benefits. Yet constant changes, including changes in processes, materials, and technology, create challenges. To minimize inefficiency, most labs have worked toward good processes and practices. All of that is still not delivering total potential value.
This research of 222 respondents from labs worldwide indicates that new technologies and processes are insufficient. Very few respondents report good – let alone excellent – performance on throughput, cycle time, documentation for compliance, or analyzing data and making sound decisions. It appears that greater integration and data standardization, a data-centric mindset, and process shift are also required.
Recommendations
Recommendations and Next Steps- Don’t rest on current processes and practices' successes; leverage new technology. You can expect excellent benefits.
- Choose technologies that will deliver the performance improvements your business most needs from the lab.
- Recognize that new technology may cause challenges for the lab. Prepare both education on the goals and training on the changes needed for each role to leverage it fully.
- Be sure you gain significant benefits from each new technology you deploy; measure and quantify gains.
- Beyond investing in new technology, be prepared to standardize and integrate it, possibly with a single platform.
- Learn about industry standards such as Allotrope and consider whether they might support and guide your efforts in making laboratory data broadly usable.
- Focus on not just having data but making it accessible and ready to aggregate, correlate, and use in new ways, such as advanced analytics.
- Ensure that data scientists and lab domain experts work together closely to generate actionable insights that deliver business value from the data.
- Realize that the data-centric mindset and approach are likely to be missing or immature, so get a top management sponsor who can support a culture shift and appropriate resources.
 What does MES need for manufacturing IT agility and to stay current in our uncertain world? It’s more than fit and functionality: a modern IT architecture. We talked to leading high-tech manufacturers to understand their vision for this.
Please enjoy the summary below. For the full paper, please visit our sponsor Critical Manufacturing (registration required).
For related research, please watch the MES Architecture for More Resilient, Responsive, and Agile Manufacturing webcast.
What does MES need for manufacturing IT agility and to stay current in our uncertain world? It’s more than fit and functionality: a modern IT architecture. We talked to leading high-tech manufacturers to understand their vision for this.
Please enjoy the summary below. For the full paper, please visit our sponsor Critical Manufacturing (registration required).
For related research, please watch the MES Architecture for More Resilient, Responsive, and Agile Manufacturing webcast.
Table of Contents
- Executive Summary
- Nothing is Stable
- Production Process Impact
- The Challenge of Enterprise MES
- Modern MES Capabilities
- Containerization for Deployment Choice
- Container Orchestration to Optimize IT Performance
- DevOps Environment for Progress
- Extending DevOps for Ecosystem Access
- Deployment Coordination
- Conclusion
- References
- About the Author
Executive Summary
Digital transformation aims to enhance agility. It must encompass every aspect of a business, including information technology (IT), business teams, and production operations, including the operations technology (OT) teams. Manufacturing execution systems (MES) and related plant floor software have been challenging to implement, maintain, and upgrade. However, manufacturers operating in uncertain times need better agility to succeed with continuous improvement and operational excellence. The ability to change quickly has become more complex. Today, it involves greater collaboration. Investment in new digital technologies, operating systems, and software applications has become a top strategic priority. This shift is now driving a digital transformation across manufacturing IT systems. Upgrading to a modern Manufacturing IT system unlocks exponential performance improvement by improving responsiveness. Manufacturers can leverage containerization strategies and DevOps environments by investing in new IT solutions with an advanced technology architecture to improve efficiency, customer satisfaction, and profit margins.FIGURE 6: OPENING DEVOPS FOR ECOSYSTEM ACCESS BEYOND THE SOFTWARE PROVIDER TO SERVICES PARTNERS AND MANUFACTURING IT TEAMS ENABLES ALL PARTIES TO KEEP THE SOFTWARE CURRENT AND OPTIMIZED
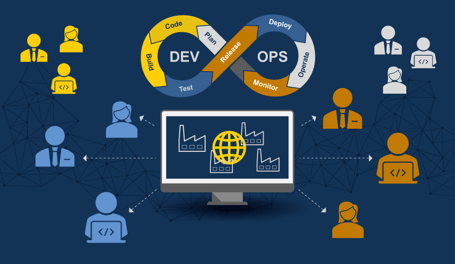 Conclusion
Conclusion
Today’s relentless pace and scope of change have forever altered how manufacturers operate. This requires companies to change the evaluation and selection process for purchasing software and systems. Companies can no longer risk staying with legacy IT systems or MES software applications in a world of extreme change and uncertainty. Even many current MES products cannot keep up.
Innovation in the software industry has yielded an array of new architectures, deployment methodologies, and hosting options. They are finally arriving on the factory floor. These advanced technologies can overcome the challenges associated with uncertainty by streamlining the configuration, standardization, and update processes related to application management. Orchestrated containerization and new DevOps platforms improve collaboration and facilitate enterprise MES deployment. This agility in architecture and tooling is fundamental to overcoming extreme uncertainty.
Manufacturers can implement a modern MES to unlock these benefits – provided it is built on a fully up-to-date architecture. With this foundation, is it possible to:
- Establish a perfect fit in every site and line, even with special one-off needs
- Manage, customize, and update applications consistently from a Center of Excellence (CoE)
- Boost performance and progress from a digital transformation strategy by using a single enterprise manufacturing software suite that is agile, responsive, and fully supports both line of business and IT needs.
Tech-Clarity Celebrates 20 Years of Making the Value of Technology Clear
 A message from Jim Brown, President of Tech-Clarity...
A message from Jim Brown, President of Tech-Clarity...
Why Tech-Clarity?
Seeing how software technology can help improve business performance in manufacturing is a fascinating thing. I had the chance to experience it from a variety of angles – in manufacturing, as a management consultant, and as a product manager and marketing leader in the software industry. Everywhere I went, I saw a consistent need. Tech people didn’t understand business people, and business people didn’t understand tech people. Somebody needed to bridge the gap in understanding between software and the business value it drives. That’s why Tech-Clarity was created with the mission to make the business value of technology clear.Time has Passed, Surely People “Get it” Now?
So much has changed over the last 20 years. Technology has evolved at a blistering pace. Business has transitioned from business process re-engineering, to lean, to digital transformation. The way people educate themselves about software has also changed, from attending conferences and reading paper reports to consuming eBooks, videos, infographics, interactive benchmarking tools, animations, and more. The web and the rise of social media put extensive information at everyone’s fingertips. Yet, even as younger, more tech-savvy generations have entered the workforce, the need – and our mission – has not changed. In fact, we believe it’s more important today than ever because business people are a more significant part of the process.What Now? Thanking Everyone (and sorry for inevitably missing some)
After 20, what I feel most is grateful. I have so much to be thankful for and so many people to thank. First, I want to thank Michelle Boucher for being the “A-Team” and helping us continuously improve. You have been the stable presence in “Team Tech-Clarity.” Thanks to Julie Fraser, who expanded our coverage as product innovation and engineering converged with manufacturing operations, and for all of the wisdom you provide. And thank you to Mandy Jiang for helping our research reach and inspire so many people. I also want to thank James White and Jeff Hojlo for the roles they played on our analyst team. I also want to express my gratitude to my team at Aberdeen Group. I learned a lot from them as Tech-Clarity was acquired to create the Product Innovation and Engineering practice. I also learned from the manufacturing team when they joined us before I left to resume the Tech-Clarity mission independently. I was fortunate to work with some talented and amazing people, many of whom have gone on to bigger and better things. Thank you to Chad Jackson for helping create and lead the practice, and to a team that always made things work, including Michelle Boucher, Risa Barnett, Dave Houlihan, Maura Buxton, Marjorie Westerman, Dave Mesgar, Adam Hollander, Scott Mitchem, Ric Stanley, and Dave Ableman. A special thanks also to Matt Littlefield, Mehul Shah, and Cindy Jutras, who put up with me as we merged our teams – we were ahead of the times! Thanks to my friends at COFES, including Brad Holtz and Joel Orr, for trusting me as a part of the team. Thanks also to Pete Wells, Lynne Allen, and Jim Doxey for teaming up on the Board of Directors to keep the spirit alive. May it rise again in some form. Thanks to our partners along the way. Thanks to my clients. There are too many of you to name, but please know how much I appreciate your support over the years. I am deeply touched and appreciative for your trust and willingness to support our mission. Thank you to the multitude of manufacturers that I’ve surveyed, interviewed, and worked with for keeping me grounded in the reality of what it takes to design and produce products amidst increasing complexity. Thanks to my family and especially my wife Cori Brown for their support and understanding of why what we do at Tech-Clarity means so much to me.Time to Get Started on the Next 20 Years
In addition to feeling grateful, I feel energized. I am excited about how digital transformation is fundamentally changing the manufacturing industry in profound ways. There are so many cool things happening in manufacturing itself and the engineering, manufacturing, and enterprise software that supports it. With the technological evolution and advances and the lines blurring between business and technology, we still need education on how tech drives better business results. The Tech-Clarity team and I are looking forward to the next 20 years!Be a Part of It
I invite you to be a part of it. Click the follow button on the Tech-Clarity, Inc. LinkedIn page. Visit our website to sign up for our newsletter and surveys. And most of all, keep us up to date on all of the cool, innovative things you’re seeing and doing. Let’s keep moving forward. There is still a lot of business value to be uncovered and realized. Thanks again for the opportunity to be a part of it.Our History in Visuals (Just for Fun)


 [post_title] => Tech-Clarity Celebrates 20 Years
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => 20-years-anniversary-tech-clarity
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:57
[post_modified_gmt] => 2022-11-15 03:25:57
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11514
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[15] => WP_Post Object
(
[ID] => 11456
[post_author] => 2
[post_date] => 2022-06-17 09:30:32
[post_date_gmt] => 2022-06-17 13:30:32
[post_content] =>
[post_title] => Tech-Clarity Celebrates 20 Years
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => 20-years-anniversary-tech-clarity
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:25:57
[post_modified_gmt] => 2022-11-15 03:25:57
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11514
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[15] => WP_Post Object
(
[ID] => 11456
[post_author] => 2
[post_date] => 2022-06-17 09:30:32
[post_date_gmt] => 2022-06-17 13:30:32
[post_content] =>  How does the right PLM help improve product development collaboration to create agility, speed, and quality in product innovation? What collaborative capabilities should companies look for when they select a PLM platform?
Products and product development have become increasingly challenging as manufacturers push the boundaries of product innovation and product development velocity. Manufacturers have to be increasingly agile to react to changing customer needs and market opportunities in the global economy. To support this, today’s product innovation process requires contributions from many roles across the enterprise and the supply chain. Efficient and effective design collaboration across departments, customers, the supply chain, and regulators is more critical than ever.
Please enjoy the summary* below. For the full research, please visit our sponsor PTC (registration required).
For related research, please read some of our previous buyer's guides, 'How to' Choose the Right PDM System, Engineering Buyer's Guide for Multi-Discipline Systems, or PLM for the Medical Device Digital Thread.
How does the right PLM help improve product development collaboration to create agility, speed, and quality in product innovation? What collaborative capabilities should companies look for when they select a PLM platform?
Products and product development have become increasingly challenging as manufacturers push the boundaries of product innovation and product development velocity. Manufacturers have to be increasingly agile to react to changing customer needs and market opportunities in the global economy. To support this, today’s product innovation process requires contributions from many roles across the enterprise and the supply chain. Efficient and effective design collaboration across departments, customers, the supply chain, and regulators is more critical than ever.
Please enjoy the summary* below. For the full research, please visit our sponsor PTC (registration required).
For related research, please read some of our previous buyer's guides, 'How to' Choose the Right PDM System, Engineering Buyer's Guide for Multi-Discipline Systems, or PLM for the Medical Device Digital Thread.
Table of Contents
- Introducing the Buyer's Guide
- The Collaboration Imperative
- What to Look for: Digital Thread
- What to Look for: Design for X
- What to Look for: Concurrent Engineering
- What to Look for: Partner Collaboration
- Considerations by Role
- Special Considerations
- Implementation and Adoption
- Vendor Considerations
- Conclusions and Next Steps
- Acknowledgments
Introducing the Buyer’s Guide
Structure of the Guide This buyer’s guide analyzes the strategic necessity of effective multi-enterprise collaboration and shares the criteria companies should consider when choosing a supporting PLM solution. The guide begins with functional requirements needed to streamline collaboration and create digital continuity across the product lifecycle. The guide looks at collaboration capabilities along four critical categories:- Supporting the digital thread
- Allowing “design for x”
- Enabling concurrent engineering
- Ensuring secure partner collaboration
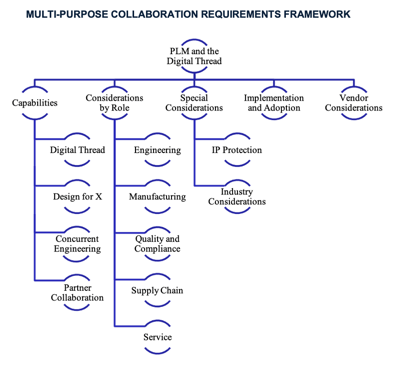
Conclusions and Next Steps
Effective Multi-Enterprise Collaboration is Mandatory Better multi-enterprise collaboration streamlines and improves product innovation processes to get the right products to market, the right way, at the right time. It helps companies get products to market faster and avoid costly, time-consuming errors. Beyond that, collaboration allows manufacturers to embrace product and product development complexity to push the boundaries of product innovation, product development speed, and agility. To operate effectively in this complex environment, manufacturers must collaborate effectively across departments, customers, the supply chain, and regulators. PLM Serves as the Backbone for Multi-Enterprise Collaboration PLM is the backbone for multi-enterprise collaboration. Companies must choose a PLM platform that can serve as the foundation to improve product development collaboration to create agility, speed, and quality in product innovation. To do this, they must select a PLM system that allows them to:- Support the digital thread
- Allow “design for x”
- Enable concurrent engineering
- Ensure secure partner collaboration
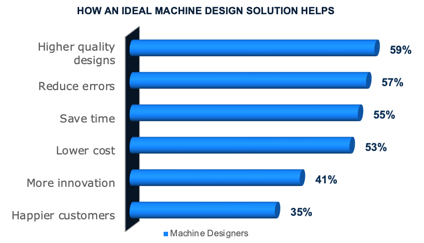
 How much better would your machine designs be if engineers wasted less time on non-value-added work?
Unfortunately, machine designs have grown so complex that engineers waste significant time on non-value-added work. Consequently, they often lack the bandwidth to meet all of these expectations. An overwhelming, 98% of machine designers report that their challenges negatively impact the business. Consequently, it can be hard to identify conflicts and find the time to fully evaluate essential engineering decisions affecting quality and cost. As a result, many struggle to avoid cost overruns and risks to delivery dates.
This research identifies six strategies machine designers can implement to reduce non-value-added work.
Please enjoy the summary below.* For the full report, please visit our sponsor Dassault Systèmes SolidWorks (registration required).
For related research, you may be interested in our other eBooks How to Reduce Non-Value-Added Work in Engineering and Industrial Design: 7 Ways to Reduce Non-Value-Added Work which provide additional insights on reducing wasted engineering effort.
How much better would your machine designs be if engineers wasted less time on non-value-added work?
Unfortunately, machine designs have grown so complex that engineers waste significant time on non-value-added work. Consequently, they often lack the bandwidth to meet all of these expectations. An overwhelming, 98% of machine designers report that their challenges negatively impact the business. Consequently, it can be hard to identify conflicts and find the time to fully evaluate essential engineering decisions affecting quality and cost. As a result, many struggle to avoid cost overruns and risks to delivery dates.
This research identifies six strategies machine designers can implement to reduce non-value-added work.
Please enjoy the summary below.* For the full report, please visit our sponsor Dassault Systèmes SolidWorks (registration required).
For related research, you may be interested in our other eBooks How to Reduce Non-Value-Added Work in Engineering and Industrial Design: 7 Ways to Reduce Non-Value-Added Work which provide additional insights on reducing wasted engineering effort.
Table of Contents
- Executive Summary
- Importance of Machine Design
- Identifying Machine Design Top Performers
- Strategies to Reduce Non-Value-Added Work
- 1. Access Product Data from Anywhere
- 2. Maximize Reuse
- 3. Improve Collaboration
- 4. Automate Machine Design Tasks
- 5. Streamline Mechanism Design Tasks
- 6. Solicit More Feedback
- Conclusion
- Recommendations
- About the Research
- Acknowledgments
The Value of Increasing Engineering Bandwidth
Imagine the Impact of Reducing Non-Value-Added Engineering Work in Machine Design How much better would your machine designs be if engineers wasted less time on non-value-added work? Engineering work is critical to competitive machine designs. However, as designs become more complex, engineering decisions become more challenging. Unfortunately, engineers waste so much time on non-value-added work, it impacts their bandwidth. Consequently, it can be hard to identify conflicts and find the time to fully evaluate essential engineering decisions affecting quality and cost. As a result, many struggle to avoid cost overruns and risks to delivery dates. About the Research Based on a survey of 228 manufacturers, this research study examines machine design practices, where engineers waste time, and best practices to avoid non-value-added work. These best practices will improve your ability to produce higher quality machines in less time, while improving your ability to meet your customers' expectations.Importance of Machine Design
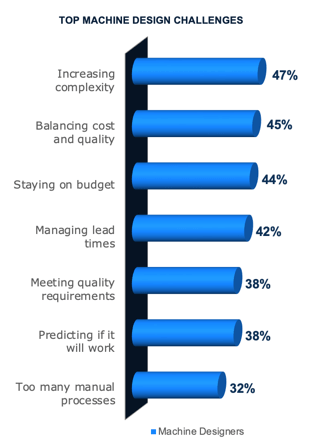 Engineers Need Empowerment
For companies that develop machines, especially industrial equipment manufacturers, engineering is critical to success. Global competition is so steep that much of what makes a machine stand out is the engineering behind it. However, many challenges hold engineers back (see graph).
Increasing Complexity
As machines become more innovative, they also get more complex. More components, smarter products, numerous configurations, mechanism calculations, and more drive product complexity. In turn, the more complex machines become, the more complicated engineering decisions get, making it difficult to balance cost and quality. These factors make predictability difficult. Plus, minor errors easily contribute to cost overruns and delays. Changes also require agility to quickly pivot and adapt without compromising lead times.
Business Impacts
Engineers Need Empowerment
For companies that develop machines, especially industrial equipment manufacturers, engineering is critical to success. Global competition is so steep that much of what makes a machine stand out is the engineering behind it. However, many challenges hold engineers back (see graph).
Increasing Complexity
As machines become more innovative, they also get more complex. More components, smarter products, numerous configurations, mechanism calculations, and more drive product complexity. In turn, the more complex machines become, the more complicated engineering decisions get, making it difficult to balance cost and quality. These factors make predictability difficult. Plus, minor errors easily contribute to cost overruns and delays. Changes also require agility to quickly pivot and adapt without compromising lead times.
Business Impacts
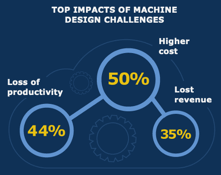 An overwhelming 98% of machine designers report that these challenges impact the business,
hurting profitability (see graph). If engineers are not more empowered, products will cost more. Meanwhile, quality issues can lead to missed delivery dates, market delays, or fewer key features, making you less competitive. Consequently, customers may go elsewhere, leading to revenue losses. At the same time, dealing with increasing complexity or addressing problems related to errors, manufacturability issues, and other difficulties hurt productivity.
Increase Engineering Bandwidth
Engineers need to be empowered to be as productive as possible to have the capacity to deal with the top machine design challenges. Unfortunately, previous Tech-Clarity research has found that engineers waste 33% of their time on non-value-added work.1 By reducing some of that time, engineers will have more bandwidth to address the top challenges of machine design.
An overwhelming 98% of machine designers report that these challenges impact the business,
hurting profitability (see graph). If engineers are not more empowered, products will cost more. Meanwhile, quality issues can lead to missed delivery dates, market delays, or fewer key features, making you less competitive. Consequently, customers may go elsewhere, leading to revenue losses. At the same time, dealing with increasing complexity or addressing problems related to errors, manufacturability issues, and other difficulties hurt productivity.
Increase Engineering Bandwidth
Engineers need to be empowered to be as productive as possible to have the capacity to deal with the top machine design challenges. Unfortunately, previous Tech-Clarity research has found that engineers waste 33% of their time on non-value-added work.1 By reducing some of that time, engineers will have more bandwidth to address the top challenges of machine design.
Conclusion
Ideal Machine Design Solution Regardless of their performance, machine designers indicate numerous business advantages to using an ideal machine design solution (see graph). While products will still get out even without an ideal solution, engineers waste so much time on non-value-added work, companies miss out on opportunities that would give them a competitive advantage. An ideal solution that supports easy access to product data from anywhere, maximizes reuse, improves collaboration, enables design automation, including mechanisms, and facilitates more opportunities for feedback will reduce this non-value-added work and free up engineering bandwidth. As a result, engineers will have more time to improve design quality, lower costs, and innovate. There will be less risk for errors, which will save time. The result will be happier customers, helping you win customer loyalty and capture market share.Recommendations
Recommendations and Next Steps By limiting the time machine designers waste on non-value-added work, you can increase engineering bandwidth, empowering them to produce higher quality designs, in less time, at a lower cost. Based on industry experience and research for this report, Tech-Clarity offers the following recommendations: Ensure machine designers can easily access product data in real-time from anywhere. This will limit their time searching for data, recreating work
because they couldn't find the data, or reworking designs due to outdated information.
Ensure machine designers can easily access product data in real-time from anywhere. This will limit their time searching for data, recreating work
because they couldn't find the data, or reworking designs due to outdated information.- Maximize reuse by making data easier to find and with a reuse library. This will save engineering time by using previously proven work, reducing the risk of introducing new errors.
- Improve collaboration by making it easier for stakeholders, especially analysts, to access design data in real-time.
- Automate machine design tasks to limit the amount of time engineers waste on tedious tasks.
- Streamline mechanism design tasks with specialized applications tailored for mechanism design that enable engineers to design mechanisms in the context of the entire assembly.
- Solicit more feedback by improving the efficiency of design reviews by extending access to all stakeholders, including customers. A cloud platform allows the flexibility to extend access to third parties and then turn it off so that you never lose control of your intellectual property.
 What are the key factors to consider when selecting warehouse management software (WMS)? As fast as supply chains are changing, how can you be ready for the future? Our new WMS Buyer’s Guide has the answers, informed by interviews with companies who have found WMS that keeps up with their business.
Please enjoy the summary below.* For the full report, please visit our sponsor Körber Supply Chain (registration required).
For related research, stay tuned for the upcoming webcast with Julie Fraser of Tech-Clarity, Kenny Williams of Parts Town, and Victoria Brown of Körber Supply Chain.
What are the key factors to consider when selecting warehouse management software (WMS)? As fast as supply chains are changing, how can you be ready for the future? Our new WMS Buyer’s Guide has the answers, informed by interviews with companies who have found WMS that keeps up with their business.
Please enjoy the summary below.* For the full report, please visit our sponsor Körber Supply Chain (registration required).
For related research, stay tuned for the upcoming webcast with Julie Fraser of Tech-Clarity, Kenny Williams of Parts Town, and Victoria Brown of Körber Supply Chain.
Table of Contents
- Introducing the Buyer’s Guide
- Flexible Flows to Meet Enterprise Needs
- Enterprise-Capable
- Enterprise IT-Capable
- Functionality
- Visibility and Optimization
- Software Technology
- Vendor Requirements
- Implementation and Service Criteria
- Optimize and Reoptimize
- Acknowledgments
- Selecting the Right WMS
Optimizing Warehouse Operations into the Unforeseeable Future
Today, many enterprises are finding their business transformation path. Companies must change, whether it's changing up suppliers or customers, moving into new product lines, regions, or markets, offering new services, or adjusting to unpredictable demand and supply situations. Competitive advantage and strategy are no longer fixed. As a result, companies need to select warehouse management systems (WMS) carefully. What are the primary considerations, and how can choosing the right WMS impact a company’s ability to succeed in their business and digital transformations today and into the future?Introducing the Buyer’s Guide
Perfect Orders in a Changing World Every industry has been suffering from disruptions and radical changes in supply, demand, and business realities. As a result, supply chain responsiveness for perfect orders is often top of mind, and enterprise warehouse management systems (WMS) are foundational to achieving that. Warehouse performance is crucial, not only for wholesale and distribution enterprises but also for retailers, e-tailers, producers, and manufacturers. Structure of the Guide This buyer’s guide describes the needs of larger enterprises to transform and continue to improve their business. It then lays out a set of critical considerations for selecting a WMS that will support the strategy now and into the future. Functionality is just the beginning of the considerations. Technology is also crucial and needs to be future-ready as the digital transformation continues. Even if the software is good, the solution provider partner or vendor plays many vital roles. To gain business value with an excellent total cost of ownership, implementation, and the ongoing relationship matter also.Optimize and Reoptimize
Get WMS that will Fit In today’s challenging supply chain environment, every company needs all of the advantages they can get. Enterprises compete on best practices they have discovered: choose a WMS designed to mold to those, and keep up as you learn, grow, and change.
Optimize the Operation
Today’s enterprise needs more from WMS than just executing the same flows repeatedly. The goal is to improve what you are doing, even as things change. The opportunity to optimize flows with simulation for the warehouse is enormous. Predictive analytics are not just a shiny buzzword but are available today in some WMS.
Future-ready
Looking ahead is the core to choosing the right WMS. Companies must prepare for what's next, whether it’s automating more, hiring new people, serving new markets, or just knowing something unforeseen will happen. Ensure your WMS is ready.
Total Cost of Ownership
Look for WMS with a good fit, self-configuration, robust integration, optimization, and a reliable software partner. All of the above adds up to a lower total cost of ownership (TCO) for an enterprise. It also points to the potential for better performance for the warehouse, the enterprise, and the entire supply chain.
*This summary is an abbreviated version of the research and does not contain the full content. For the full research, please visit our sponsor Körber Supply Chain (registration required).
If you have difficulty obtaining a copy of the report, please contact us.
[post_title] => Warehouse Management Systems (WMS) (buyer's guide)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => warehouse-management-systems-buyers-guide
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:28:03
[post_modified_gmt] => 2022-11-15 03:28:03
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11471
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[18] => WP_Post Object
(
[ID] => 11447
[post_author] => 2
[post_date] => 2022-06-13 09:00:12
[post_date_gmt] => 2022-06-13 13:00:12
[post_content] =>
In today’s challenging supply chain environment, every company needs all of the advantages they can get. Enterprises compete on best practices they have discovered: choose a WMS designed to mold to those, and keep up as you learn, grow, and change.
Optimize the Operation
Today’s enterprise needs more from WMS than just executing the same flows repeatedly. The goal is to improve what you are doing, even as things change. The opportunity to optimize flows with simulation for the warehouse is enormous. Predictive analytics are not just a shiny buzzword but are available today in some WMS.
Future-ready
Looking ahead is the core to choosing the right WMS. Companies must prepare for what's next, whether it’s automating more, hiring new people, serving new markets, or just knowing something unforeseen will happen. Ensure your WMS is ready.
Total Cost of Ownership
Look for WMS with a good fit, self-configuration, robust integration, optimization, and a reliable software partner. All of the above adds up to a lower total cost of ownership (TCO) for an enterprise. It also points to the potential for better performance for the warehouse, the enterprise, and the entire supply chain.
*This summary is an abbreviated version of the research and does not contain the full content. For the full research, please visit our sponsor Körber Supply Chain (registration required).
If you have difficulty obtaining a copy of the report, please contact us.
[post_title] => Warehouse Management Systems (WMS) (buyer's guide)
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => warehouse-management-systems-buyers-guide
[to_ping] =>
[pinged] =>
[post_modified] => 2022-11-14 22:28:03
[post_modified_gmt] => 2022-11-15 03:28:03
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11471
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[18] => WP_Post Object
(
[ID] => 11447
[post_author] => 2
[post_date] => 2022-06-13 09:00:12
[post_date_gmt] => 2022-06-13 13:00:12
[post_content] =>  How are manufacturers improving manufacturing engineering performance with better practices and technology? Join this webcast as Jim Brown previews survey results from our 3D and Virtual Simulation in Manufacturing Engineering study.
Companies are under increasing pressure to deliver more complex and personalized products to market faster to meet consumer demands. As the pace of design, engineering and manufacturing climbs, so does the challenge of maintaining high levels of quality without incurring additional cost and delays.
The virtual build process focuses on technology and process that can bridge the gap that exists between engineering and manufacturing and enable companies to achieve their goals of satisfying customer demand and capturing market share without sacrificing profit or quality.
Join Tech-Clarity’s Jim Brown and Dassault Systemes Delmia’s Strategic Business Development Director Adrian Wood in a discussion about how manufacturing engineering is evolving.
How are manufacturers improving manufacturing engineering performance with better practices and technology? Join this webcast as Jim Brown previews survey results from our 3D and Virtual Simulation in Manufacturing Engineering study.
Companies are under increasing pressure to deliver more complex and personalized products to market faster to meet consumer demands. As the pace of design, engineering and manufacturing climbs, so does the challenge of maintaining high levels of quality without incurring additional cost and delays.
The virtual build process focuses on technology and process that can bridge the gap that exists between engineering and manufacturing and enable companies to achieve their goals of satisfying customer demand and capturing market share without sacrificing profit or quality.
Join Tech-Clarity’s Jim Brown and Dassault Systemes Delmia’s Strategic Business Development Director Adrian Wood in a discussion about how manufacturing engineering is evolving.
 Register now for the final webcast of this three part series hosted by Dassault Systemes Delmia.
Click here to watch the first webcast in this series, also featuring Jim Brown and Adrian Wood.
[post_title] => The Future of Manufacturing Engineering
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => manufacturing-engineering-webcast-2
[to_ping] =>
[pinged] =>
[post_modified] => 2023-12-15 17:25:07
[post_modified_gmt] => 2023-12-15 22:25:07
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11447
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[19] => WP_Post Object
(
[ID] => 11439
[post_author] => 2
[post_date] => 2022-06-09 09:00:07
[post_date_gmt] => 2022-06-09 13:00:07
[post_content] =>
Register now for the final webcast of this three part series hosted by Dassault Systemes Delmia.
Click here to watch the first webcast in this series, also featuring Jim Brown and Adrian Wood.
[post_title] => The Future of Manufacturing Engineering
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => manufacturing-engineering-webcast-2
[to_ping] =>
[pinged] =>
[post_modified] => 2023-12-15 17:25:07
[post_modified_gmt] => 2023-12-15 22:25:07
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11447
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[19] => WP_Post Object
(
[ID] => 11439
[post_author] => 2
[post_date] => 2022-06-09 09:00:07
[post_date_gmt] => 2022-06-09 13:00:07
[post_content] =>  There is a clear, industry-wide transition of software solutions to the cloud, but not all solutions are moving at the same pace. Cloud PLM adoption has accelerated, but how quickly are companies transitioning? And how can they make sure that they continue to drive business value from PLM while enjoying the benefits of cloud computing?
Register for this webcast on Thursday, June 30th at 11am ET to hear Tech-Clarity’s Jim Brown share previews from his recent survey on the State of Cloud PLM 2022 and Aras’ Bruce Bookbinder as he offers insights from his conversations with customers. Join us for this interactive discussion.
[post_title] => How Fast is PLM Moving to the Cloud?
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-plm-webcast-2
[to_ping] =>
[pinged] =>
[post_modified] => 2023-12-15 17:20:23
[post_modified_gmt] => 2023-12-15 22:20:23
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11439
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
)
[post_count] => 20
[current_post] => -1
[before_loop] => 1
[in_the_loop] =>
[post] => WP_Post Object
(
[ID] => 11730
[post_author] => 2574
[post_date] => 2022-08-17 22:50:08
[post_date_gmt] => 2022-08-18 02:50:08
[post_content] =>
There is a clear, industry-wide transition of software solutions to the cloud, but not all solutions are moving at the same pace. Cloud PLM adoption has accelerated, but how quickly are companies transitioning? And how can they make sure that they continue to drive business value from PLM while enjoying the benefits of cloud computing?
Register for this webcast on Thursday, June 30th at 11am ET to hear Tech-Clarity’s Jim Brown share previews from his recent survey on the State of Cloud PLM 2022 and Aras’ Bruce Bookbinder as he offers insights from his conversations with customers. Join us for this interactive discussion.
[post_title] => How Fast is PLM Moving to the Cloud?
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => cloud-plm-webcast-2
[to_ping] =>
[pinged] =>
[post_modified] => 2023-12-15 17:20:23
[post_modified_gmt] => 2023-12-15 22:20:23
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11439
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
)
[post_count] => 20
[current_post] => -1
[before_loop] => 1
[in_the_loop] =>
[post] => WP_Post Object
(
[ID] => 11730
[post_author] => 2574
[post_date] => 2022-08-17 22:50:08
[post_date_gmt] => 2022-08-18 02:50:08
[post_content] =>  How do you bridge the gulf between product engineering and manufacturing? In the product lifecycle, manufacturing sits at the center. Rapid product and process improvement, lower cost of quality, and business innovation rest on engineering and manufacturing working together effectively. Yet often they don’t or can’t.
Tech-Clarity research shows that MES and PLM are the top two applications Top Performers use to manage manufacturing data. Yet, like the disciplines, they often have a gap between them. That break in the digital thread limits companies’ ability to be truly agile, to innovate at top quality and at minimal cost.
Register to hear iBASEt’s Attila Labas, Razorleaf’s Jonathan Scott, and Tech-Clarity’s Julie Fraser as they discuss the issues and challenges of PLM-MES gap bridging. Download now to hear this recording of the live webinar.
[post_title] => The PLM – MES Gap and Why Bridging it is Urgent Now
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => plm-mes-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-02 13:19:32
[post_modified_gmt] => 2024-01-02 18:19:32
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11730
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[comment_count] => 0
[current_comment] => -1
[found_posts] => 875
[max_num_pages] => 44
[max_num_comment_pages] => 0
[is_single] =>
[is_preview] =>
[is_page] =>
[is_archive] =>
[is_date] =>
[is_year] =>
[is_month] =>
[is_day] =>
[is_time] =>
[is_author] =>
[is_category] =>
[is_tag] =>
[is_tax] =>
[is_search] =>
[is_feed] =>
[is_comment_feed] =>
[is_trackback] =>
[is_home] => 1
[is_privacy_policy] =>
[is_404] =>
[is_embed] =>
[is_paged] =>
[is_admin] =>
[is_attachment] =>
[is_singular] =>
[is_robots] =>
[is_favicon] =>
[is_posts_page] =>
[is_post_type_archive] =>
[query_vars_hash:WP_Query:private] => 49e8e426bcbc3c5d523212feda09adc3
[query_vars_changed:WP_Query:private] => 1
[thumbnails_cached] =>
[allow_query_attachment_by_filename:protected] =>
[stopwords:WP_Query:private] =>
[compat_fields:WP_Query:private] => Array
(
[0] => query_vars_hash
[1] => query_vars_changed
)
[compat_methods:WP_Query:private] => Array
(
[0] => init_query_flags
[1] => parse_tax_query
)
[query_cache_key:WP_Query:private] => wp_query:c18d9dd6a2d153717185ccf54d72b0a6:0.04720700 17685460050.05639900 1768546005
)
How do you bridge the gulf between product engineering and manufacturing? In the product lifecycle, manufacturing sits at the center. Rapid product and process improvement, lower cost of quality, and business innovation rest on engineering and manufacturing working together effectively. Yet often they don’t or can’t.
Tech-Clarity research shows that MES and PLM are the top two applications Top Performers use to manage manufacturing data. Yet, like the disciplines, they often have a gap between them. That break in the digital thread limits companies’ ability to be truly agile, to innovate at top quality and at minimal cost.
Register to hear iBASEt’s Attila Labas, Razorleaf’s Jonathan Scott, and Tech-Clarity’s Julie Fraser as they discuss the issues and challenges of PLM-MES gap bridging. Download now to hear this recording of the live webinar.
[post_title] => The PLM – MES Gap and Why Bridging it is Urgent Now
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => plm-mes-webcast
[to_ping] =>
[pinged] =>
[post_modified] => 2024-01-02 13:19:32
[post_modified_gmt] => 2024-01-02 18:19:32
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://tech-clarity.com/?p=11730
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[comment_count] => 0
[current_comment] => -1
[found_posts] => 875
[max_num_pages] => 44
[max_num_comment_pages] => 0
[is_single] =>
[is_preview] =>
[is_page] =>
[is_archive] =>
[is_date] =>
[is_year] =>
[is_month] =>
[is_day] =>
[is_time] =>
[is_author] =>
[is_category] =>
[is_tag] =>
[is_tax] =>
[is_search] =>
[is_feed] =>
[is_comment_feed] =>
[is_trackback] =>
[is_home] => 1
[is_privacy_policy] =>
[is_404] =>
[is_embed] =>
[is_paged] =>
[is_admin] =>
[is_attachment] =>
[is_singular] =>
[is_robots] =>
[is_favicon] =>
[is_posts_page] =>
[is_post_type_archive] =>
[query_vars_hash:WP_Query:private] => 49e8e426bcbc3c5d523212feda09adc3
[query_vars_changed:WP_Query:private] => 1
[thumbnails_cached] =>
[allow_query_attachment_by_filename:protected] =>
[stopwords:WP_Query:private] =>
[compat_fields:WP_Query:private] => Array
(
[0] => query_vars_hash
[1] => query_vars_changed
)
[compat_methods:WP_Query:private] => Array
(
[0] => init_query_flags
[1] => parse_tax_query
)
[query_cache_key:WP_Query:private] => wp_query:c18d9dd6a2d153717185ccf54d72b0a6:0.04720700 17685460050.05639900 1768546005
)
All Results for "All"
Choosing the Right Cloud SaaS PLM
What do manufacturers need to look for as they plan to adopt cloud PLM? Our updated buyer’s guide shares requirements to help manufacturers ensure that their cloud SaaS PLM solution will meet their product innovation and digital transformation needs. Please enjoy the summary* below. For the full research, please visit our sponsor PTC (registration required). For…
The State of Cloud PLM
Companies are making a significant shift to the cloud, how is that impacting PLM? What do manufacturers need to be aware of related to features, customization, and upgrades as they make the transition? Our survey results include insights from over one hundred manufacturers to find out. Please enjoy the summary below.* For the full report,…
Improving Continuous Improvement
Should you be improving continuous improvement (CI) in your company? For many companies, the answer is yes. Find out the difference between a typical continuous improvement program and one that’s better in this infographic to see whether you have room for improvement on your CI. Data is at the heart of any CI program. Yet…
Future of CPG PLM with Kalypso and Pepsico
What does the future look like for PLM in the CPG industry? Tech-Clarity led an interactive discussion with Pepsico and Kalypso PLM leaders in a Kalypso-hosted webinar. It was an interactive discussion with PLM industry leaders from a variety of roles to discuss the findings of our recent research on the Future of PLM in…
Strategic Sourcing: Leverage AI to Mitigate Risk and Cut Costs (quote spotlight)
How can strategic sourcing professionals lower risk and cost in these uncertain times? This new paper by Procurement Leaders points to the potential of AI in a marketplace, and quotes Tech-Clarity’s Julie Fraser. Please enjoy the summary* below. For the full research, please visit Procurement Leaders (registration required). Materials price increases and supplier risk have…
Three Tips to Ensure IIoT Delivers Business Value
What can manufacturers do to gain full business value from IIoT investments? Julie Fraser shares three tips to create a firm foundation for success on The Peggy Smedley Show podcast. Hear four examples of how new technology infrastructure and architecture can support bridging the IT/OT divide. Plus a focus on process improvement and people. All…
PLM ROI Payback
What can you expect for PLM ROI? How will it help your business? Engineers waste a lot of valuable time on non-value-added work. However, our research finds that Product Lifecycle Management (PLM) can help. It provides business value by empowering engineers to focus more of their valuable time on innovation, design, and engineering. Still, how…
Adopting a Model-Based Enterprise (MBE) Strategy?
What should you consider to ensure your MBE strategy is successful? Manufacturers adopting a Model-Based Enterprise (MBE) strategy report improved traceability and agility, faster delivery, greater efficiency, and lower costs. What should you consider to realize similar benefits? An MBE is an organization that uses digital models to support commissioning, operating, servicing, and decommissioning a…
Julie Fraser on The Manufacturing IT Podcast with Daniel Langley
What has changed in the past 30 years of manufacturing? Listen to the Manufacturing IT Podcast episode where Julie Fraser shares her thoughts with host Daniel Langley. We wander through topics from cigars and sexism to rebranding manufacturing and remaking companies to be more customer-centric and serious about ESG to overcome the skills shortage. You’ll…
A New Era of Manufacturing Continuous Improvement
Has the time come to do continuous improvement (CI) on the approach to CI? We think so. There is a new era in manufacturing, so it’s time for a new era in continuous improvement programs. We interviewed manufacturers, consultants, and associations to validate the notion we explain in this white paper. Please enjoy the summary*…
Enhancing Customer Experience with Your Supply Chain Strategy
Customer expectations for rapid, complete shipments are rising constantly. How can a business set supply chain strategy to meet current and future needs? Listen to this webcast from July 19, 2022 where Julie Fraser explores this with Kenny William of Parts Town and Victoria Brown of Körber Supply Chain. Both have deep experience in…
New Technology in The Lab
Beyond implementing lab technologies, what can laboratories do to leverage them effectively? We surveyed 222 people working in corporate labs to find out. Please enjoy the summary* below. For the full research, please visit our sponsor Dassault Systemès BIOVIA (registration required). Table of Contents Current Situation Challenges Technology – The Solution? Additional Needs Recommendations About…
Digital Transformation for Manufacturing Agility
What does MES need for manufacturing IT agility and to stay current in our uncertain world? It’s more than fit and functionality: a modern IT architecture. We talked to leading high-tech manufacturers to understand their vision for this. Please enjoy the summary below. For the full paper, please visit our sponsor Critical Manufacturing (registration required)….
Tech-Clarity Celebrates 20 Years
Tech-Clarity Celebrates 20 Years of Making the Value of Technology Clear A message from Jim Brown, President of Tech-Clarity… Tech-Clarity recently turned 20 years old. I have to admit that it snuck up on me. I didn’t think much about it until I started getting “congratulations on your work anniversary” messages on LinkedIn. Two decades…
Choosing PLM to Support Collaboration (eBook)
How does the right PLM help improve product development collaboration to create agility, speed, and quality in product innovation? What collaborative capabilities should companies look for when they select a PLM platform? Products and product development have become increasingly challenging as manufacturers push the boundaries of product innovation and product development velocity. Manufacturers have to…
Machine Design: How to Reduce Non-Value-Added Work
How much better would your machine designs be if engineers wasted less time on non-value-added work? Unfortunately, machine designs have grown so complex that engineers waste significant time on non-value-added work. Consequently, they often lack the bandwidth to meet all of these expectations. An overwhelming, 98% of machine designers report that their challenges negatively impact…
Warehouse Management Systems (WMS) (buyer’s guide)
What are the key factors to consider when selecting warehouse management software (WMS)? As fast as supply chains are changing, how can you be ready for the future? Our new WMS Buyer’s Guide has the answers, informed by interviews with companies who have found WMS that keeps up with their business. Please enjoy the summary…
The Future of Manufacturing Engineering
How are manufacturers improving manufacturing engineering performance with better practices and technology? Join this webcast as Jim Brown previews survey results from our 3D and Virtual Simulation in Manufacturing Engineering study. Companies are under increasing pressure to deliver more complex and personalized products to market faster to meet consumer demands. As the pace of design,…
How Fast is PLM Moving to the Cloud?
There is a clear, industry-wide transition of software solutions to the cloud, but not all solutions are moving at the same pace. Cloud PLM adoption has accelerated, but how quickly are companies transitioning? And how can they make sure that they continue to drive business value from PLM while enjoying the benefits of cloud computing? …
 What do manufacturers need to look for as they plan to adopt cloud PLM? Our updated buyer’s guide shares requirements to help manufacturers ensure that their cloud SaaS PLM solution will meet their product innovation and digital transformation needs.
What do manufacturers need to look for as they plan to adopt cloud PLM? Our updated buyer’s guide shares requirements to help manufacturers ensure that their cloud SaaS PLM solution will meet their product innovation and digital transformation needs.